Label: XACDURO- sulbactam and durlobactam kit
- NDC Code(s): 68547-111-10, 68547-211-20, 68547-311-30
- Packager: La Jolla Pharmaceutical Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use XACDURO safely and effectively. See full prescribing information for XACDURO. XACDURO® (sulbactam for injection; durlobactam for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP) XACDURO is indicated in patients 18 years of age and older for the treatment of ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - XACDURO is a co-packaged product containing sulbactam for injection and durlobactam for injection. The recommended dosage of XACDURO is 1 gram (g) of sulbactam and 1 g ...
-
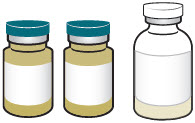
3 DOSAGE FORMS AND STRENGTHSXACDURO is a co-packaged kit containing the following two components as sterile powders for reconstitution: 1 clear single-dose vial of sulbactam for injection 1g (as white to off-white powder ...
-
4 CONTRAINDICATIONSXACDURO is contraindicated in patients with a history of known severe hypersensitivity to the components of XACDURO (sulbactam and durlobactam), or other beta-lactam antibacterial drugs [see ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Serious and occasionally fatal hypersensitivity (anaphylactic) reactions and serious skin reactions have been reported in patients receiving beta-lactam ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described in greater detail in the Warnings and Precautions section: Hypersensitivity Reactions [see Warnings and Precautions (5.1)] Clostridioides ...
-
7 DRUG INTERACTIONS7.1 Organic Anion Transporter 1 (OAT1) Inhibitors - Concomitant administration with OAT1 inhibitors may increase plasma concentrations of sulbactam. Concomitant administration of OAT1 inhibitors ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - XACDURO - There are no available data on the use of XACDURO in pregnancy to evaluate for a drug-associated risk of major birth defects, miscarriage, or other ...
-
10 OVERDOSAGEThere is no information on the clinical signs and symptoms associated with an overdose of XACDURO. Neurological adverse reactions, including convulsions, may occur with the attainment of high CSF ...
-
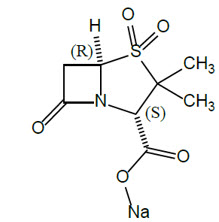
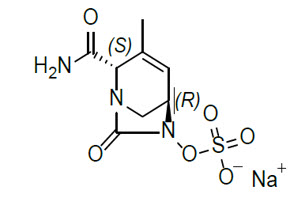
11 DESCRIPTION XACDURO (sulbactam for injection and durlobactam for injection) is an antibacterial co-packaged product containing sulbactam sodium, a penicillin derivative beta-lactam antibacterial and ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - XACDURO is an antibacterial drug [see Microbiology (12.4)]. 12.2 Pharmacodynamics - For sulbactam, the percent time of dosing interval that unbound plasma ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Carcinogenicity studies with XACDURO have not been conducted. Mutagenesis - Durlobactam was negative in genetic ...
-
14 CLINICAL STUDIESTreatment of Hospital-acquired and Ventilator-associated Bacterial Pneumonia Caused by Acinetobacter baumannii-calcoaceticus Complex Organisms - A total of 177 hospitalized adults with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - XACDURO is a co-packaged product containing sulbactam for injection and durlobactam for injection. XACDURO is supplied as a kit (NDC 68547-111-10) containing the following ...
-
17 PATIENT COUNSELING INFORMATIONSerious Allergic Reactions - Advise the patient, their families, or caregivers that allergic reactions, including serious allergic reactions, could occur and that serious reactions require ...
-
SPL UNCLASSIFIED SECTIONDistributed by: La Jolla Pharmaceutical Company, Waltham, MA 02451 - XACDURO is a registered trademark of Entasis Therapeutics Ltd. Copyright ©2023, Entasis Therapeutics Ltd. All rights reserved.
-
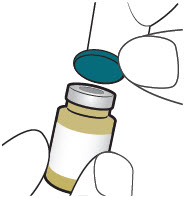
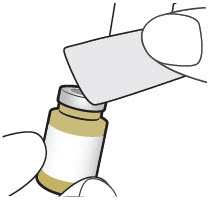
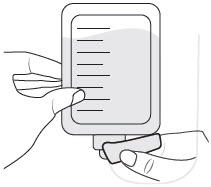
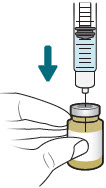
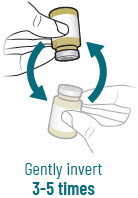
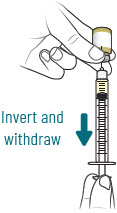
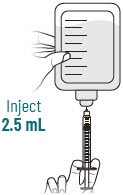
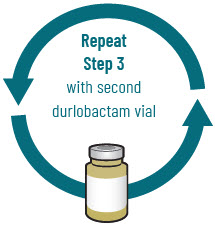
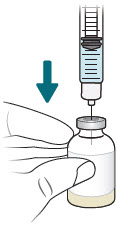
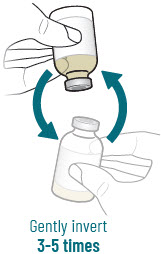
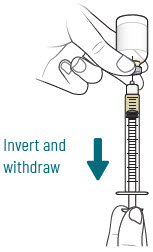
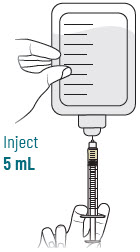
INSTRUCTIONS FOR USEXACDURO® (sulbactam for injection; durlobactam for injection), co-packaged for intravenous use - 1 g/1 g per dose - Single-dose kit - 3 vialsDurlobactam - 0.5 g/vial - Durlobactam - 0.5 ...
-
PRINCIPAL DISPLAY PANEL - Kit CartonSterile - For intravenous infusion after dilution - XACDURO® (sulbactam for injection; durlobactam for injection), co-packaged for intravenous use - Durlobactam must be - administered with ...
-
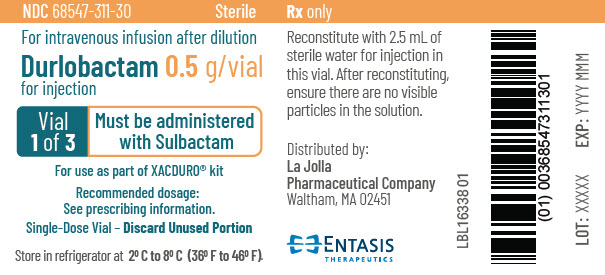
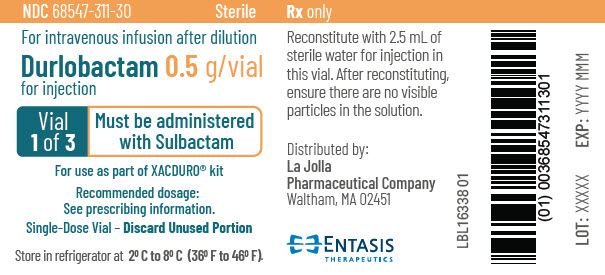
PRINCIPAL DISPLAY PANEL - 0.5 g Vial Label - 1 of 3NDC 68547-311-30 - Sterile - For intravenous infusion after dilution - Durlobactam 0.5 g/vial - for injection - Vial - 1 of 3 - Must be administered - with Sulbactam - For use as part of XACDURO® kit - Recommended ...
-
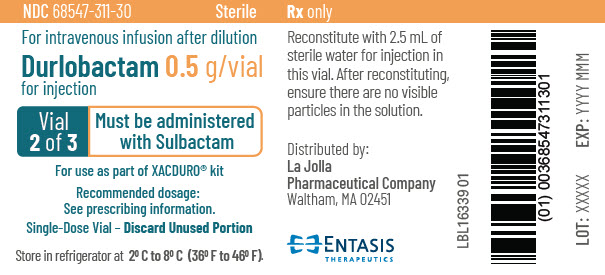
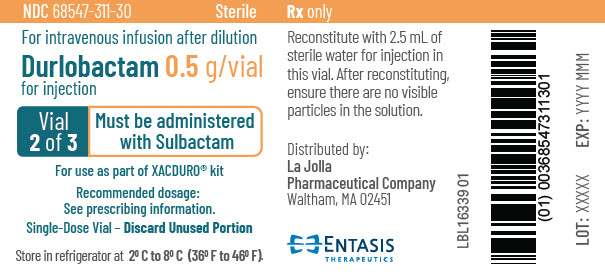
PRINCIPAL DISPLAY PANEL - 0.5 g Vial Label - 2 of 3NDC 68547-311-30 - Sterile - For intravenous infusion after dilution - Durlobactam 0.5 g/vial - for injection - Vial - 2 of 3 - Must be administered - with Sulbactam - For use as part of XACDURO® kit - Recommended ...
-
PRINCIPAL DISPLAY PANEL - 1 g Vial LabelNDC 68547-211-20 - Sterile - For intravenous infusion after dilution - Sulbactam 1 g/vial - for injection - Vial - 3 of 3 - Must be administered - with Durlobactam - For use as part of XACDURO® kit - Recommended ...
-
INGREDIENTS AND APPEARANCEProduct Information