Label: LEVOCARNITINE tablet
- NDC Code(s): 70954-492-10
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
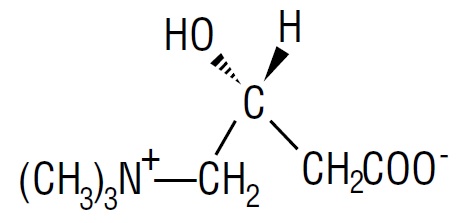
DESCRIPTIONLevocarnitine is a carrier molecule in the transport of long-chain fatty acids across the inner mitochondrial membrane. The chemical name of levocarnitine is 3-carboxy-2(R)-hydroxy-N, N ...
-
CLINICAL PHARMACOLOGYLevocarnitine is a naturally occurring substance required in mammalian energy metabolism. It has been shown to facilitate long-chain fatty acid entry into cellular mitochondria, thereby delivering ...
-
PHARMACOKINETICSIn a relative bioavailability study in 15 healthy adult male volunteers, Levocarnitine Tablets were found to be bio-equivalent to Levocarnitine Oral Solution. Following 4 days of dosing with 6 ...
-
METABOLISM AND EXCRETIONIn a pharmacokinetic study where five normal adult male volunteers received an oral dose of [3H-methyl]-L-carnitine following 15 days of a high carnitine diet and additional carnitine supplement ...
-
INDICATIONS & USAGELevocarnitine is indicated in the treatment of primary systemic carnitine deficiency. In the reported cases, the clinical presentation consisted of recurrent episodes of Reye-like encephalopathy ...
-
CONTRAINDICATIONSNone known.
-
WARNINGSHypersensitivity Reactions - Serious hypersensitivity reactions, including rash, urticaria, and facial edema have been reported with oral Levocarnitine. Other serious hypersensitivity reactions ...
-
PRECAUTIONSGENERAL PRECAUTIONS - Levocarnitine Oral Solution and Levocarnitine Oral Solution (Sugar Free) are for oral/internal use only. Not for parenteral use. Gastrointestinal reactions may ...
-
ADVERSE REACTIONSThe following adverse reactions associated with the use of oral formulations of levocarnitine were identified in clinical trials or post-marketing reports. Because these reactions were reported ...
-
OVERDOSAGEThere have been no reports of toxicity from levocarnitine overdosage. Levocarnitine is easily removed from plasma by dialysis. The intravenous LD50 of levocarnitine in rats is 5.4 g/kg and the ...
-
DOSAGE & ADMINISTRATIONLevocarnitine Tablets. Adults: The recommended oral dosage for adults is 990 mg two or three times a day using the 330 mg tablets, depending on clinical response. Infants and children: The ...
-
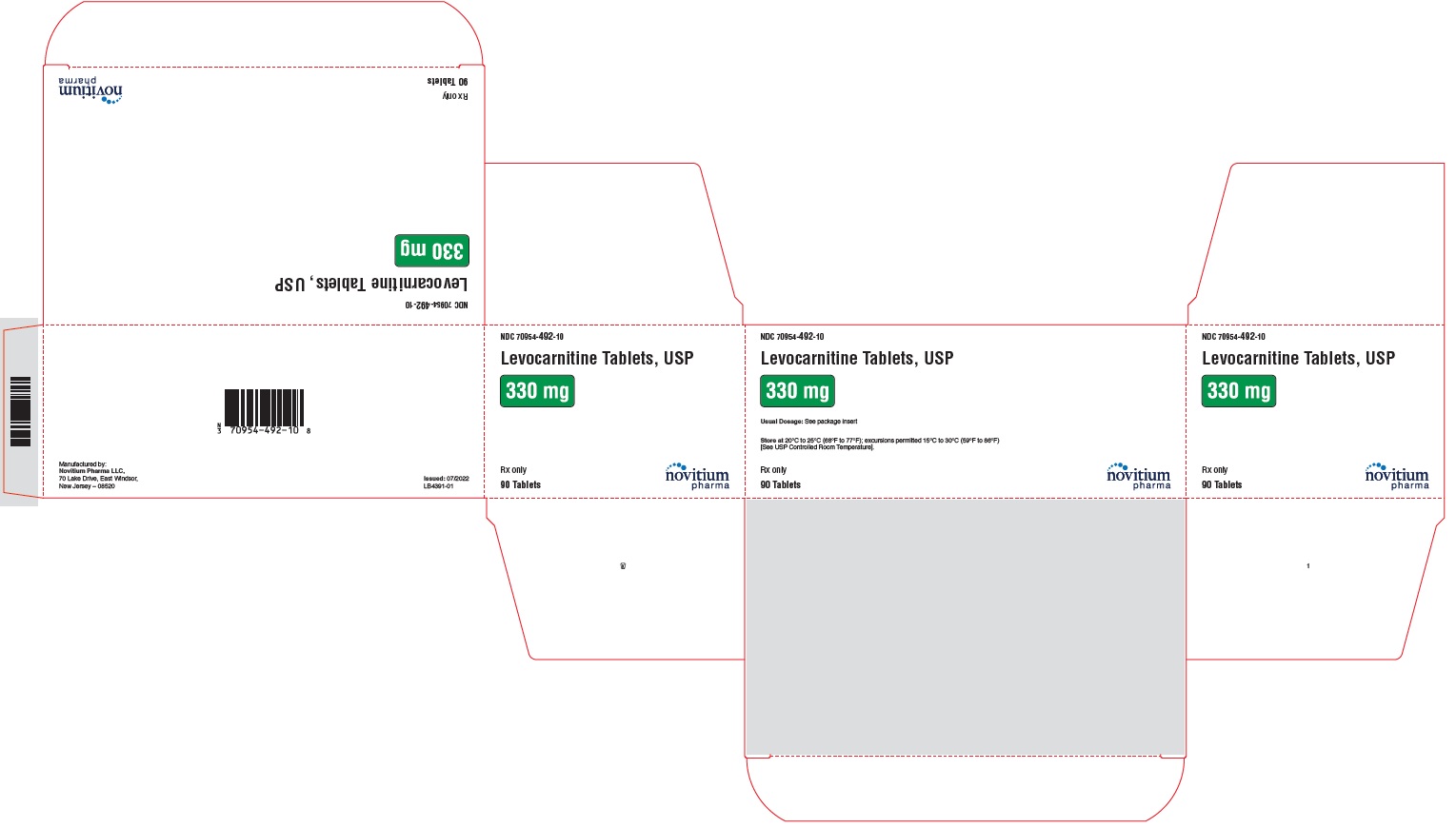
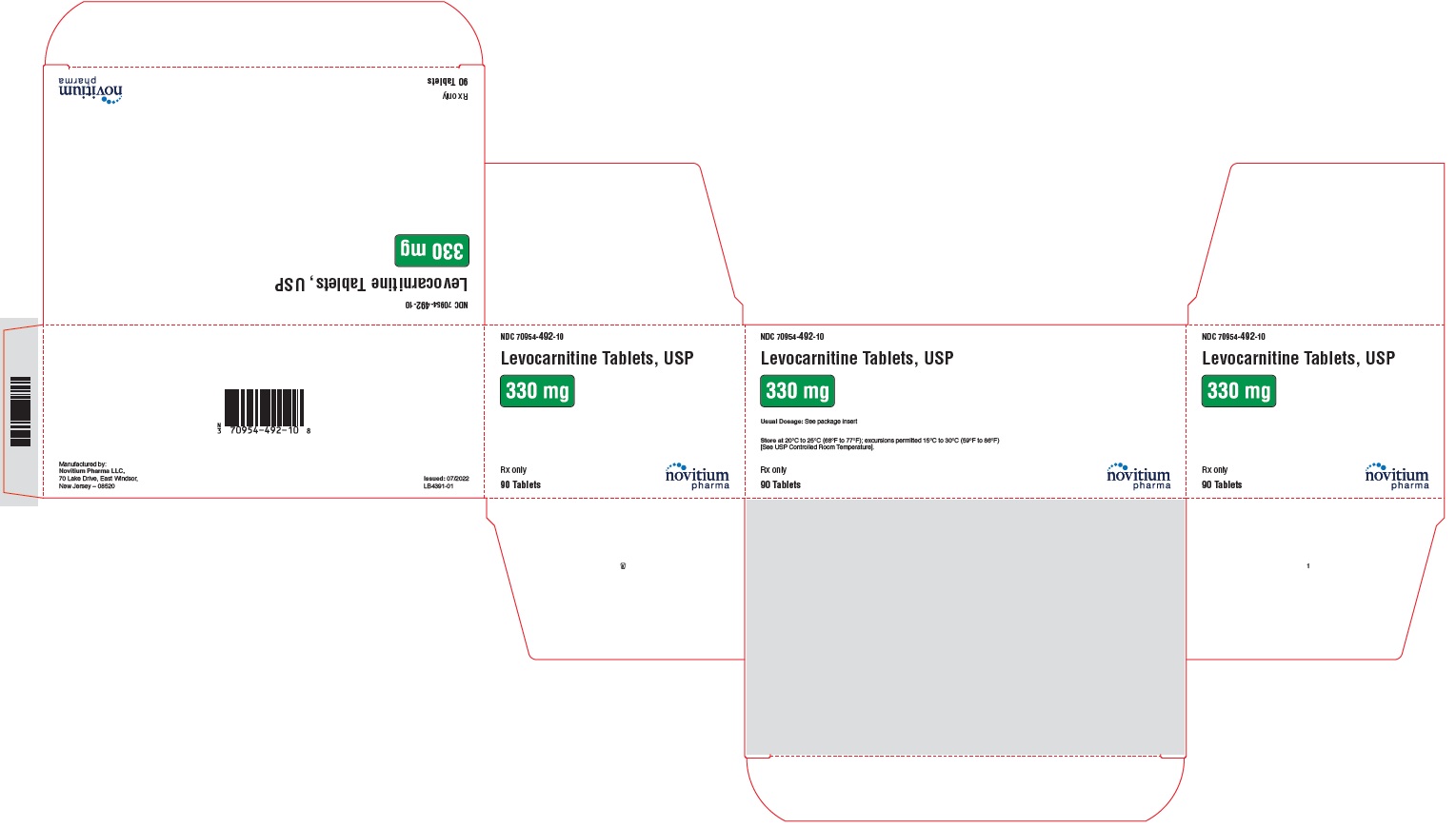
HOW SUPPLIEDLevocarnitine Tablets, USP are supplied as 330 mg white to off white, round, standard biconvex, uncoated tablets debossed with N492 on one side and plain on the other side in individual blisters ...
-
REFERENCESBohmer, T., Rydning, A. and Solberg, H.E. 1974. Carnitine levels in human serum in health and disease. Clin. Chim. Acta 57:55-61. Brooks, H., Goldberg, L., Holland, R. et al. 1977 ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELLevocarnitine Tablets, USP 330 mg - NDC 70954-492-10 - 90 tablets per carton
-
INGREDIENTS AND APPEARANCEProduct Information