Label: DAYTIME COLD AND FLU RELIEF- acetaminohpen, dextromethorphan hbr, guaifenesin phenylephrine hcl liquid
- NDC Code(s): 0363-7270-12
- Packager: WALGREENS CO.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
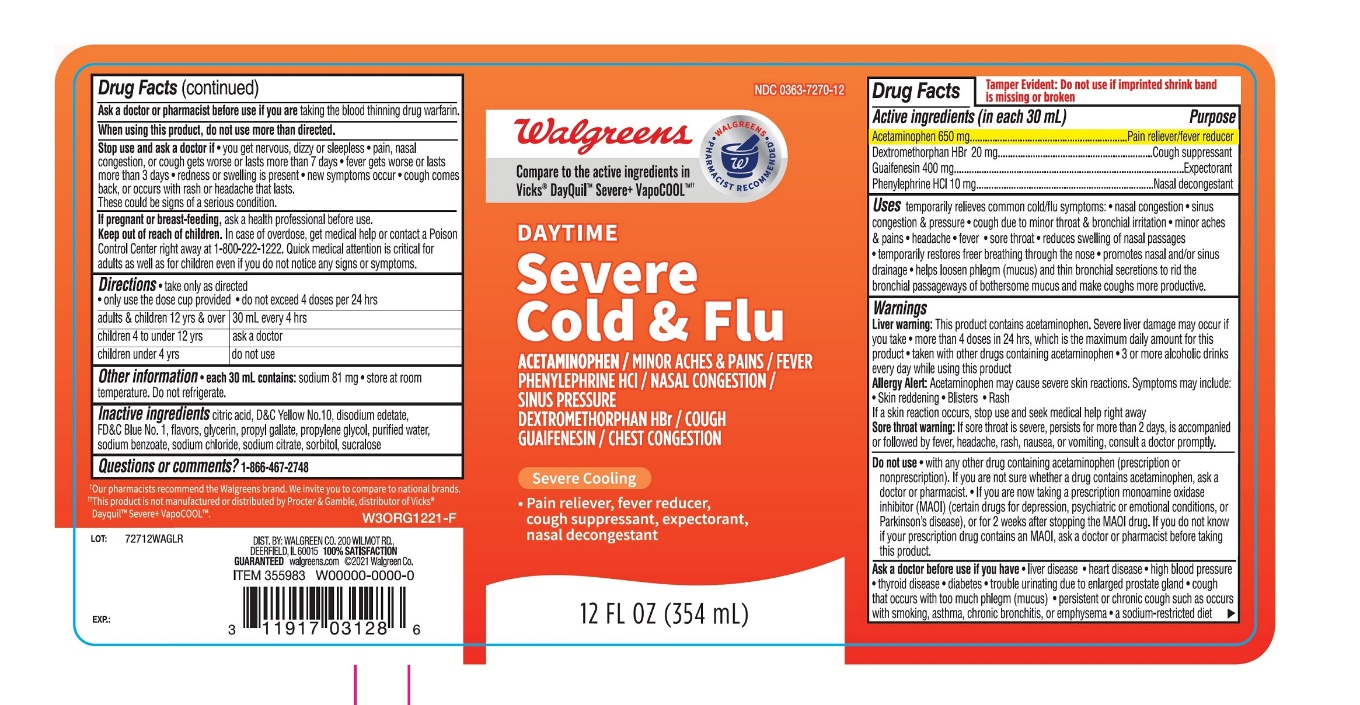
- Active ingredients (in each 30 mL)
- Purposes
-
Uses
- •
- temporarily relieves common cold/flu symptoms:
- •
- nasal congestion
- •
- sinus congestion & pressure
- •
- cough due to minor throat and bronchial irritation
- •
- minor aches and pains
- •
- headache
- •
- fever
- •
- sore throat
- •
- reduce swelling of nasal passages
- •
- temporarily restores freer breathing through the nose
- •
- promotes nasal and/or sinus drainage
- •
- help loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make cough more productive
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- •
- more than 4 doses in 24 hrs which is maximum daily amount for this product
- •
- taken with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- Skin reddening
- •
- Blisters
- •
- Rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- •
- with any other drug containing acetaminophen (prescription or non-prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease) or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- liver disease
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
- •
- cough that occurs with too much phlegm (mucus)
- •
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- •
- a sodium-restricted diet
Stop use and ask a doctor if
- •
- you get nervous, dizzy, or sleepless
- •
- pain, nasal congestion or cough gets worse, or lasts more than 7 days
- •
- fever gets worse or lasts more than 3 days
- •
- redness or swelling is present
- •
- new symptoms occur
- •
- cough comes back or occurs with a rash or headache that lasts. These could be signs of a serious condition.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
Compare to the active ingredients in VICKS® Dayquil™ Severe+ VapoCOOL™ *
NDC 0363-7270-12
Day Time
Cold & Flu Relief
Acetaminophen - Pain Reliever/Fever Reducer
Dextromethorphan HBr - Cough Suppressant
Phenylephrine HCI - Nasal decongestant
For Relieves of
Minor Aches & Pains, Fever
Nasal congestion & Sinus Pressure
Cough
Chest congestion
12 FL OZ (354 mL)
DO NOT USE IF IMPRINTED SHRINK BAND IS MISSING OR BROKEN
DISTRIBUTED BY
*This product.is not manufactured or distributed by Procter & Gamble, distributer of Vicks® Dayquil™ Severe+ VapoCOOL™.
-
INGREDIENTS AND APPEARANCE
DAYTIME COLD AND FLU RELIEF
acetaminohpen, dextromethorphan hbr, guaifenesin phenylephrine hcl liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-7270 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg in 30 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 30 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg in 30 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg in 30 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GLYCERIN (UNII: PDC6A3C0OX) PROPYL GALLATE (UNII: 8D4SNN7V92) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-7270-12 354 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/02/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 02/02/2021 Labeler - WALGREENS CO. (008965063)