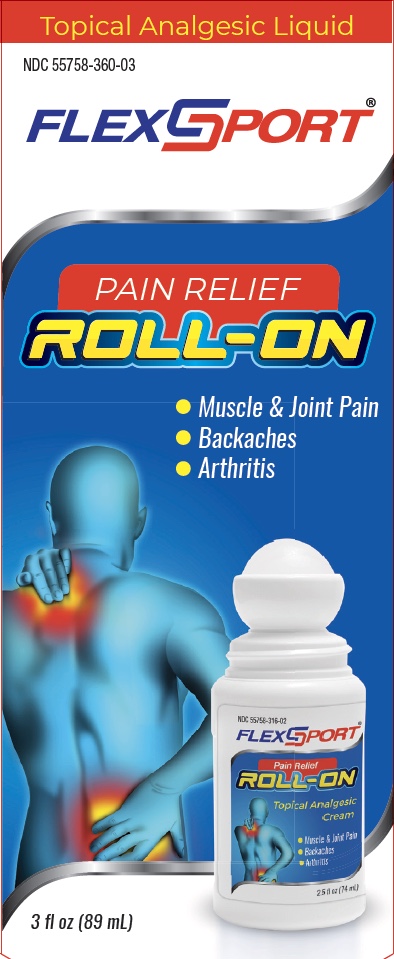
Label: FLEXSPORT ROLL ON- methyl salicylate liquid
- NDC Code(s): 55758-360-03
- Packager: Pharmadel LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient and Purpose
- Uses
-
Warnings
For external use only. Avoid contact with the eyes.
Flammable: keep away from fire or flame.
When using this product
- do not bandage tightly or use with a heating pad
- do not apply to wounds or damaged, broken or irritated skin
- Directions
- Other information
- Inactive ingredients
- Questions or Comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
FLEXSPORT ROLL ON
methyl salicylate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55758-360 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 15 g in 100 g Inactive Ingredients Ingredient Name Strength CAPSAICIN (UNII: S07O44R1ZM) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) MENTHOL (UNII: L7T10EIP3A) POLYSORBATE 20 (UNII: 7T1F30V5YH) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) DMDM HYDANTOIN (UNII: BYR0546TOW) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) TROLAMINE (UNII: 9O3K93S3TK) EUCALYPTUS OIL (UNII: 2R04ONI662) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55758-360-03 1 in 1 BOX 04/04/2021 1 89 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 04/04/2021 Labeler - Pharmadel LLC (030129680)