Label: CARBAMAZEPINE ER tablet, extended release
- NDC Code(s): 62135-927-60, 62135-928-60, 62135-930-60
- Packager: Chartwell RX, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNINGS
SERIOUS DERMATOLOGIC REACTIONS AND HLA-B*1502 ALLELE
SERIOUS AND SOMETIMES FATAL DERMATOLOGIC REACTIONS, INCLUDING TOXIC EPIDERMAL NECROLYSIS (TEN) AND STEVENS-JOHNSON SYNDROME (SJS), HAVE BEEN REPORTED DURING TREATMENT WITH CARBAMAZEPINE. THESE REACTIONS ARE ESTIMATED TO OCCUR IN 1 TO 6 PER 10,000 NEW USERS IN COUNTRIES WITH MAINLY CAUCASIAN POPULATIONS, BUT THE RISK IN SOME ASIAN COUNTRIES IS ESTIMATED TO BE ABOUT 10 TIMES HIGHER. STUDIES IN PATIENTS OF CHINESE ANCESTRY HAVE FOUND A STRONG ASSOCIATION BETWEEN THE RISK OF DEVELOPING SJS/TEN AND THE PRESENCE OF HLA-B*1502, AN INHERITED ALLELIC VARIANT OF THE HLA-B GENE. HLA-B*1502 IS FOUND ALMOST EXCLUSIVELY IN PATIENTS WITH ANCESTRY ACROSS BROAD AREAS OF ASIA. PATIENTS WITH ANCESTRY IN GENETICALLY AT-RISK POPULATIONS SHOULD BE SCREENED FOR THE PRESENCE OF HLA-B*1502 PRIOR TO INITIATING TREATMENT WITH CARBAMAZEPINE. PATIENTS TESTING POSITIVE FOR THE ALLELE SHOULD NOT BE TREATED WITH CARBAMAZEPINE UNLESS THE BENEFIT CLEARLY OUTWEIGHS THE RISK (SEE WARNINGSAND PRECAUTIONS, LABORATORY TESTS).
APLASTIC ANEMIA AND AGRANULOCYTOSIS
APLASTIC ANEMIA AND AGRANULOCYTOSIS HAVE BEEN REPORTED IN ASSOCIATION WITH THE USE OF CARBAMAZEPINE. DATA FROM A POPULATION-BASED CASE CONTROL STUDY DEMONSTRATE THAT THE RISK OF DEVELOPING THESE REACTIONS IS 5 TO 8 TIMES GREATER THAN IN THE GENERAL POPULATION. HOWEVER, THE OVERALL RISK OF THESE REACTIONS IN THE UNTREATED GENERAL POPULATION IS LOW, APPROXIMATELY SIX PATIENTS PER ONE MILLION POPULATION PER YEAR FOR AGRANULOCYTOSIS AND TWO PATIENTS PER ONE MILLION POPULATION PER YEAR FOR APLASTIC ANEMIA.
ALTHOUGH REPORTS OF TRANSIENT OR PERSISTENT DECREASED PLATELET OR WHITE BLOOD CELL COUNTS ARE NOT UNCOMMON IN ASSOCIATION WITH THE USE OF CARBAMAZEPINE, DATA ARE NOT AVAILABLE TO ESTIMATE ACCURATELY THEIR INCIDENCE OR OUTCOME. HOWEVER, THE VAST MAJORITY OF THE CASES OF LEUKOPENIA HAVE NOT PROGRESSED TO THE MORE SERIOUS CONDITIONS OF APLASTIC ANEMIA OR AGRANULOCYTOSIS.
BECAUSE OF THE VERY LOW INCIDENCE OF AGRANULOCYTOSIS AND APLASTIC ANEMIA, THE VAST MAJORITY OF MINOR HEMATOLOGIC CHANGES OBSERVED IN MONITORING OF PATIENTS ON CARBAMAZEPINE ARE UNLIKELY TO SIGNAL THE OCCURRENCE OF EITHER ABNORMALITY. NONETHELESS, COMPLETE PRETREATMENT HEMATOLOGICAL TESTING SHOULD BE OBTAINED AS A BASELINE. IF A PATIENT IN THE COURSE OF TREATMENT EXHIBITS LOW OR DECREASED WHITE BLOOD CELL OR PLATELET COUNTS, THE PATIENT SHOULD BE MONITORED CLOSELY. DISCONTINUATION OF THE DRUG SHOULD BE CONSIDERED IF ANY EVIDENCE OF SIGNIFICANT BONE MARROW DEPRESSION DEVELOPS
Close -
SPL UNCLASSIFIED SECTIONBefore prescribing carbamazepine, the physician should be thoroughly familiar with the details of this prescribing information, particularly regarding use with other drugs, especially those which ...
-
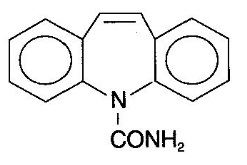
DESCRIPTIONCarbamazepine USP, is an anticonvulsant and specific analgesic for trigeminal neuralgia, available for oral administration as extended-release tablets of 100, 200, and 400 mg. Its chemical name is ...
-
CLINICAL PHARMACOLOGYIn controlled clinical trials, carbamazepine has been shown to be effective in the treatment of psychomotor and grand mal seizures, as well as trigeminal neuralgia.
-
Mechanism of ActionCarbamazepine has demonstrated anticonvulsant properties in rats and mice with electrically and chemically induced seizures. It appears to act by reducing polysynaptic responses and blocking the ...
-
PharmacokineticsIn clinical studies, carbamazepine suspension, conventional tablets, and extended-release tablets delivered equivalent amounts of drug to the systemic circulation. However, the suspension was ...
- INDICATIONS AND USAGE
-
EpilepsyCarbamazepine is indicated for use as an anticonvulsant drug. Evidence supporting efficacy of carbamazepine as an anticonvulsant was derived from active drug-controlled studies that enrolled ...
-
Trigeminal NeuralgiaCarbamazepine is indicated in the treatment of the pain associated with true trigeminal neuralgia. Beneficial results have also been reported in glossopharyngeal neuralgia. This drug is not a ...
-
CONTRAINDICATIONSCarbamazepine should not be used in patients with a history of previous bone marrow depression, hypersensitivity to the drug, or known sensitivity to any of the tricyclic compounds, such as ...
- WARNINGS
-
Serious Dermatologic ReactionsSerious and sometimes fatal dermatologic reactions, including toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS), have been reported with carbamazepine treatment. The risk of ...
-
SJS/TEN and HLA-B*1502 AlleleRetrospective case-control studies have found that in patients of Chinese ancestry there is a strong association between the risk of developing SJS/TEN with carbamazepine treatment and the ...
-
Hypersensitivity Reactions and HLA-A*3101 AlleleRetrospective case-control studies in patients of European, Korean, and Japanese ancestry have found a moderate association between the risk of developing hypersensitivity reactions and the ...
-
Aplastic Anemia and AgranulocytosisAplastic anemia and agranulocytosis have been reported in association with the use of carbamazepine (see - BOXED WARNING). Patients with a history of adverse hematologic reaction to any drug may ...
-
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/ Multiorgan HypersensitivityDrug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan hypersensitivity, has occurred with carbamazepine. Some of these events have been fatal or life-threatening ...
-
HypersensitivityHypersensitivity reactions to carbamazepine have been reported in patients who previously experienced this reaction to anticonvulsants including phenytoin, primidone, and phenobarbital. If such ...
-
Anaphylaxis and AngioedemaRare cases of anaphylaxis and angioedema involving the larynx, glottis, lips, and eyelids have been reported in patients after taking the first or subsequent doses of carbamazepine. Angioedema ...
-
Suicidal Behavior and IdeationAntiepileptic drugs (AEDs), including carbamazepine, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any ...
-
GeneralCarbamazepine has shown mild anticholinergic activity that may be associated with increased intraocular pressure; therefore, patients with increased intraocular pressure should be closely observed ...
-
Usage in PregnancyCarbamazepine can cause fetal harm when administered to a pregnant woman. Epidemiological data suggest that there may be an association between the use of carbamazepine during pregnancy and ...
- PRECAUTIONS
-
GeneralBefore initiating therapy, a detailed history and physical examination should be made. Carbamazepine should be used with caution in patients with a mixed seizure disorder that includes atypical ...
-
Information for PatientsPatients should be informed of the availability of a Medication Guide and they should be instructed to read the Medication Guide before taking carbamazepine. Patients should be made aware of the ...
-
Laboratory TestsFor genetically at-risk patients (see - WARNINGS), high-resolution ‘ HLA-B*1502 typing’ is recommended. The test is positive if either one or two HLA-B*1502 alleles are detected and negative ...
-
Drug InteractionsThere has been a report of a patient who passed an orange rubbery precipitate in his stool the day after ingesting carbamazepine suspension immediately followed by Thorazine - ®*solution ...
-
Agents That May Affect Carbamazepine Plasma LevelsWhen carbamazepine is given with drugs that can increase or decrease carbamazepine levels, close monitoring of carbamazepine levels is indicated and dosage adjustment may be required. Agents That ...
-
Effect of Carbamazepine on Plasma Levels of Concomitant AgentsDecreased Levels of Concomitant Medications - Carbamazepine is a potent inducer of hepatic 3A4 and is also known to be an inducer of CYP1A2, 2B6, 2C9/19, and may therefore reduce plasma ...
-
Other Drug InteractionsCyclophosphamide is an inactive prodrug and is converted to its active metabolite in part by CYP3A. The rate of metabolism and the leukopenic activity of cyclophosphamide are reportedly increased ...
-
Carcinogenesis, Mutagenesis, Impairment of FertilityCarbamazepine, when administered to Sprague-Dawley rats for two years in the diet at doses of 25, 75, and 250 mg/kg/day, resulted in a dose-related increase in the incidence of hepatocellular ...
-
Usage in Pregnancy(see - WARNINGS).
-
Labor and DeliveryThe effect of carbamazepine on human labor and delivery is unknown.
-
Nursing MothersCarbamazepine and its epoxide metabolite are transferred to breast milk. The ratio of the concentration in breast milk to that in maternal plasma is about 0.4 for carbamazepine and about 0.5 for ...
-
Pediatric UseSubstantial evidence of carbamazepine’s effectiveness for use in the management of children with epilepsy (see - INDICATIONS AND USAGEfor specific seizure types) is derived from clinical ...
-
Geriatric UseNo systematic studies in geriatric patients have been conducted.
-
ADVERSE REACTIONSIf adverse reactions are of such severity that the drug must be discontinued, the physician must be aware that abrupt discontinuation of any anticonvulsant drug in a responsive epileptic patient ...
-
DRUG ABUSE AND DEPENDENCENo evidence of abuse potential has been associated with carbamazepine, nor is there evidence of psychological or physical dependence in humans.
- OVERDOSAGE
-
Acute ToxicityLowest known lethal dose: adults, 3.2 g (a 24-year-old woman died of a cardiac arrest and a 24-year-old man died of pneumonia and hypoxic encephalopathy); children, 4 g (a 14-year-old girl died of ...
-
Signs and SymptomsThe first signs and symptoms appear after 1 to 3 hours. Neuromuscular disturbances are the most prominent. Cardiovascular disorders are generally milder, and severe cardiac complications occur ...
-
TreatmentThe prognosis in cases of severe poisoning is critically dependent upon prompt elimination of the drug, which may be achieved by inducing vomiting, irrigating the stomach, and by taking ...
-
DOSAGE AND ADMINISTRATION (SEE TABLE BELOW)Carbamazepine suspension in combination with liquid chlorpromazine or thioridazine results in precipitate formation, and, in the case of chlorpromazine, there has been a report of a patient ...
-
Epilepsy (SEE INDICATIONS AND USAGE)Adults and children over 12 years of age-Initial:Either 200 mg twice a day for tablets and extended-release tablets, or 1 teaspoon four times a day for suspension (400 mg/day). Increase at weekly ...
-
Trigeminal Neuralgia (SEE INDICATIONS AND USAGE)Initial:On the first day, either 100 mg twice a day for tablets or extended-release tablets, or ½ teaspoon four times a day for suspension, for a total daily dose of 200 mg. This daily dose may ...
-
HOW SUPPLIEDCarbamazepine Extended-release tablets, USP100 mg-round, yellow, coated (imprinted CER 100 on one side), release portal on one side - Bottles of 60 ...
-
MEDICATION GUIDEPrint Medication Guides at ...
-
PRINCIPAL DISPLAY PANELCarbamazepine Extended-Release Tablets, USP 100 mg - NDC 62135-927-60 60sBottle Label - Carbamazepine Extended-Release Tablets, USP 200 mg - NDC 62135-928-60 60sBottle Label - Carbamazepine ...
-
INGREDIENTS AND APPEARANCEProduct Information