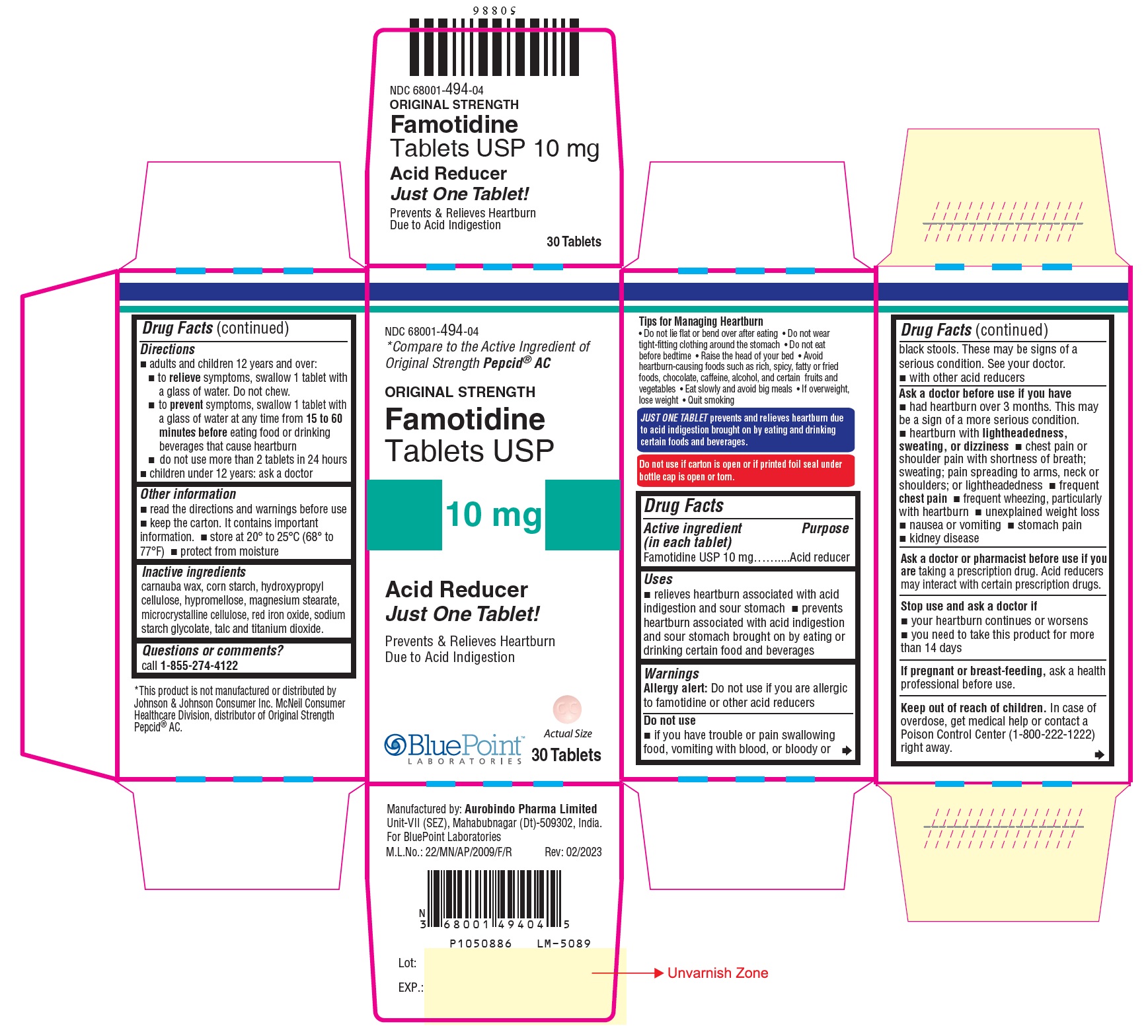
Label: FAMOTIDINE tablet
- NDC Code(s): 68001-494-04, 68001-494-06
- Packager: BluePoint Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient in (Each Tablet)
- Purpose
- Uses
- Warnings
- Do not use
-
Ask a Doctor Before Use if You Have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- kidney disease
- Ask a Doctor or Pharmacist Before Use if You Are
- Stop Use and ask a Doctor if
- If Pregnant or Breast-Feeding
- Keep out of reach of children
-
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- to prevent symptoms, swallow 1 tablet with a glass of water at any time from 15 to 60 minutes before eating food or drinking beverages that cause heartburn
- do not use more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
- Other Information
- Inactive Ingredients
-
Questions or Comments?
call 1-855-274-4122
Tips for Managing Heartburn
- Do not lie flat or bend over after eating
- Do not wear tight-fitting clothing around the stomach
- Do not eat before bedtime
- Raise the head of your bed
- Avoid heartburn-causing foods such as rich, spicy, fatty or fried foods, chocolate, caffeine, alcohol, and certain fruits and vegetables
- Eat slowly and avoid big meals
- If overweight, lose weight
- Quit smoking
JUST ONE TABLET prevents and relieves heartburn due to acid indigestion brought on by eating and drinking certain foods and beverages.
Do not use if carton is open or if printed foil seal under bottle cap is open or torn.Manufactured by:
Aurobindo Pharma Limited
Unit-VII (SEZ), Mahabubnagar (Dt)-509302, India .For BluePoint Laboratories
Rev: 02/2023
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
FAMOTIDINE
famotidine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68001-494 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FAMOTIDINE (UNII: 5QZO15J2Z8) (FAMOTIDINE - UNII:5QZO15J2Z8) FAMOTIDINE 10 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) CARNAUBA WAX (UNII: R12CBM0EIZ) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) TALC (UNII: 7SEV7J4R1U) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color pink Score no score Shape ROUND (biconvex) Size 5mm Flavor Imprint Code CC;58 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68001-494-04 1 in 1 CARTON 04/07/2021 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:68001-494-06 1 in 1 CARTON 06/17/2021 2 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206531 04/07/2021 Labeler - BluePoint Laboratories (985523874) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650381903 manufacture(68001-494) , analysis(68001-494)