Label: FELODIPINE tablet, film coated
-
NDC Code(s):
51407-087-01,
51407-087-05,
51407-088-01,
51407-088-05, view more51407-089-01, 51407-089-05
- Packager: Golden State Medical Supply, Inc.
- This is a repackaged label.
- Source NDC Code(s): 61442-431, 61442-432, 61442-433
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Felodipine is a calcium antagonist (calcium channel blocker). Felodipine is a
dihydropyridine derivative that is chemically described as ± ethyl methyl
4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate.
Its molecular formula is C18H19Cl12NO4 and its structural formula is: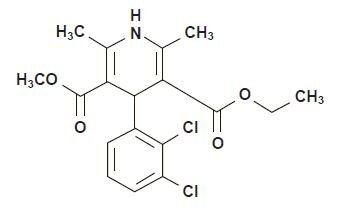
Felodipine, USP is a light yellow to yellow crystalline powder with a molecular
weight of 384.26. It is insoluble in water and is freely soluble in acetone and
in methanol; very slightly soluble in heptane. Felodipine is a racemic mixture.
Felodipine extended-release tablets, USP provide extended release of felodipine.
They are available as tablets containing 2.5 mg, 5 mg or 10 mg of felodipine,
USP for oral administration. Inactive ingredients are: lactose monohydrate,
hydroxypropyl cellulose, silicon dioxide colloidal, hypromellose, magnesium
stearate, calcium phosphate dibasic, butylated hydroxyanisole, polyethylene
glycol, titanuium dioxide. In addition, the 5 mg and the 10 mg tablet strength
contain FD&C Red No. 40 powder, FD&C Yellow No. 6 Aluminum Lake.
Meets USP Dissolution Test 3. -
CLINICAL PHARMACOLOGY
Mechanism of Action
Felodipine is a member of the dihydropyridine class of calcium channel
antagonists (calcium channel blockers). It reversibly competes with nitrendipine
and/or other calcium channel blockers for dihydropyridine binding sites,
blocks voltage-dependent Ca++ currents in vascular smooth muscle and
cultured rabbit atrial cells, and blocks potassium-induced contracture of the
rat portal vein.
In vitro studies show that the effects of felodipine on contractile processes
are selective, with greater effects on vascular smooth muscle than cardiac
muscle. Negative inotropic effects can be detected in vitro, but such effects
have not been seen in intact animals.
The effect of felodipine on blood pressure is principally a consequence of a
dose related decrease of peripheral vascular resistance in man, with a modest
reflex increase in heart rate (see Cardiovascular Effects). With the exception
of a mild diuretic effect seen in several animal species and man, the effects
of felodipine are accounted for by its effects on peripheral vascular resistance.Pharmacokinetics and Metabolism
Following oral administration, felodipine is almost completely absorbed and
undergoes extensive first-pass metabolism. The systemic bioavailability of
felodipine extended-release tablets is approximately 20%. Mean peak
concentrations following the administration of felodipine extended-release
tablets are reached in 2.5 to 5 hours. Both peak plasma concentration and the
area under the plasma concentration time curve (AUC) increase linearly with
doses up to 20 mg. Felodipine is greater than 99% bound to plasma proteins.
Following intravenous administration, the plasma concentration of felodipine
declined triexponentially with mean disposition half-lives of 4.8 minutes,
1.5 hours, and 9.1 hours. The mean contributions of the three individual
phases to the overall AUC were 15%, 40% and 45%, respectively, in the order
of increasing t1/2.
Following oral administration of the immediate-release formulation, the plasma
level of felodipine also declined polyexponentially with a mean terminal t1/2 of
11 to 16 hours. The mean peak and trough steady-state plasma concentrations
achieved after 10 mg of the immediate-release formulation given once a day
to normal volunteers, were 20 and 0.5 nmol/L, respectively. The trough plasma
concentration of felodipine in most individuals was substantially below the
concentration needed to effect a half-maximal decline in blood pressure (EC50)
[4 to 6 nmol/L for felodipine], thus precluding once a day dosing with the
immediate-release formulation.
Following administration of a 10 mg dose of felodipine, the extended-release
formulation, to young, healthy volunteers, mean peak and trough steady-state
plasma concentrations of felodipine were 7 and 2 nmol/L, respectively.
Corresponding values in hypertensive patients (mean age 64) after a 20 mg
dose of felodipine extended-release tablets were 23 and 7 nmol/L. Since the
EC50 for felodipine is 4 to 6 nmol/L, a 5 mg to 10 mg dose of felodipine
extended-release tablets in some patients, and a 20 mg dose in others, would
be expected to provide an antihypertensive effect that persists for 24 hours
(see Cardiovascular Effects and DOSAGE AND ADMINISTRATION).
The systemic plasma clearance of felodipine in young healthy subjects is
about 0.8 L/min, and the apparent volume of distribution is about 10 L/kg.
Following an oral or intravenous dose of 14C-labeled felodipine in man, about
70% of the dose of radioactivity was recovered in urine and 10% in the feces.
A negligible amount of intact felodipine is recovered in the urine and feces
(< 0.5%). Six metabolites, which account for 23% of the oral dose, have been
identified; none has significant vasodilating activity.
Following administration of felodipine extended-release tablets to hypertensive
patients, mean peak plasma concentrations at steady-state are about 20%
higher than after a single dose. Blood pressure response is correlated with
plasma concentrations of felodipine.
The bioavailability of felodipine extended-release tablets is influenced by the
presence of food. When administered either with a high fat or carbohydrate
diet, Cmax is increased by approximately 60%; AUC is unchanged. When
felodipine extended-release tablets were administered after a light meal
(orange juice, toast, and cereal), however, there is no effect on felodipine's
pharmacokinetics. The bioavailability of felodipine was increased approximately
2-fold when taken with grapefruit juice. Orange juice does not appear
to modify the kinetics of felodipine extended-release tablets. A similar finding
has been seen with other dihydropyridine calcium antagonists, but to a lesser
extent than that seen with felodipine.Geriatric Use
Plasma concentrations of felodipine, after a single dose and at steady-state,
increase with age. Mean clearance of felodipine in elderly hypertensives
(mean age 74) was only 45% of that of young volunteers (mean age 26).
At steady-state mean AUC for young patients was 39% of that for the elderly.
Data for intermediate age ranges suggest that the AUCs fall between the
extremes of the young and the elderly.Hepatic Dysfunction
In patients with hepatic disease, the clearance of felodipine was reduced to
about 60% of that seen in normal young volunteers.
Renal impairment does not alter the plasma concentration profile of felodipine;
although higher concentrations of the metabolites are present in the plasma
due to decreased urinary excretion, these are inactive.
Animal studies have demonstrated that felodipine crosses the blood-brain
barrier and the placenta.Cardiovascular Effects
Following administration of felodipine extended-release tablets, a reduction
in blood pressure generally occurs within 2 to 5 hours. During chronic
administration, substantial blood pressure control lasts for 24 hours, with
trough reductions in diastolic blood pressure approximately 40% to 50% of
peak reductions. The antihypertensive effect is dose dependent and correlates
with the plasma concentration of felodipine.
A reflex increase in heart rate frequently occurs during the first week of
therapy; this increase attenuates over time. Heart rate increases of 5 to
10 beats per minute may be seen during chronic dosing. The increase is
inhibited by beta-blocking agents.
The P-R interval of the ECG is not affected by felodipine when administered
alone or in combination with a beta-blocking agent. Felodipine alone or in
combination with a beta-blocking agent has been shown, in clinical and
electrophysiologic studies, to have no significant effect on cardiac conduction
(P-R, P-Q, and H-V intervals).
In clinical trials in hypertensive patients without clinical evidence of left ventricular
dysfunction, no symptoms suggestive of a negative inotropic effect were
noted; however, none would be expected in this population (see PRECAUTIONS).Renal/Endocrine Effects
Renal vascular resistance is decreased by felodipine while glomerular filtration
rate remains unchanged. Mild diuresis, natriuresis, and kaliuresis have been
observed during the first week of therapy. No significant effects on serum
electrolytes were observed during short- and long-term therapy.
In clinical trials in patients with hypertension, increases in plasma noradrenaline
levels have been observed.Clinical Studies
Felodipine produces dose related decreases in systolic and diastolic blood
pressure as demonstrated in six placebo-controlled, dose response studies
using either immediate-release or extended-release dosage forms. These
studies enrolled over 800 patients on active treatment, at total daily doses
ranging from 2.5 mg to 20 mg. In those studies felodipine was administered
either as monotherapy or was added to beta-blockers. The results of the two
studies with felodipine extended-release tablets given once daily as monotherapy
are shown in the table below: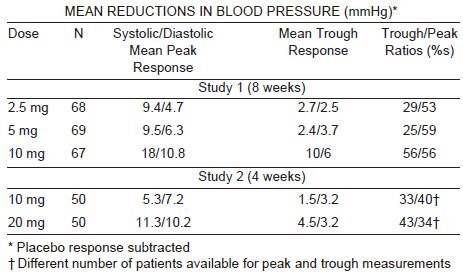
-
INDICATIONS AND USAGE
Felodipine extended-release tablets, USP are indicated for the treatment of
hypertension, to lower blood pressure. Lowering blood pressure lowers the
risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial
infarctions. These benefits have been seen in controlled trials of
antihypertensive drugs from a wide variety of pharmacologic classes including
felodipine.
Control of high blood pressure should be part of comprehensive cardiovascular
risk management, including, as appropriate, lipid control, diabetes management,
antithrombotic therapy, smoking cessation, exercise and limited sodium intake.
Many patients will require more than one drug to achieve blood pressure
goals. For specific advice on goals and management, see published guidelines,
such as those of the National High Blood Pressure Education Program’s Joint
National Committee on Prevention, Detection, Evaluation, and Treatment of
High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes
and with different mechanisms of action, have been shown in randomized
controlled trials to reduce cardiovascular morbidity and mortality, and it can be
concluded that it is blood pressure reduction, and not some other pharmacologic
property of the drugs, that is largely responsible for those benefits. The largest
and most consistent cardiovascular outcome benefit has been a reduction in
the risk of stroke, but reductions in myocardial infarction and cardiovascular
mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk,
and the absolute risk increase per mmHg is greater at higher blood pressures,
so that even modest reductions of severe hypertension can provide substantial
benefit. Relative risk reduction from blood pressure reduction is similar across
populations with varying absolute risk, so the absolute benefit is greater in
patients who are at higher risk independent of their hypertension (for example,
patients with diabetes or hyperlipidemia), and such patients would be expected
to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as
monotherapy) in black patients, and many antihypertensive drugs have
additional approved indications and effects (e.g., on angina, heart failure or
diabetic kidney disease). These considerations may guide selection of therapy.
Felodipine extended-release tablets, USP may be administered with other
antihypertensive agents. - CONTRAINDICATIONS
-
PRECAUTIONS
General
Hypotension
Felodipine, like other calcium antagonists, may occasionally precipitate
significant hypotension and, rarely, syncope. It may lead to reflex tachycardia
which in susceptible individuals may precipitate angina pectoris. (See
ADVERSE REACTIONS.)
Heart Failure
Although acute hemodynamic studies in a small number of patients with
NYHA Class II or III heart failure treated with felodipine have not demonstrated
negative inotropic effects, safety in patients with heart failure has not
been established. Caution, therefore, should be exercised when using
ffelodipine extended-release tablets in patients with heart failure or compromised
ventricular function, particularly in combination with a beta-blocker.
Patients with Impaired Liver Function
Patients with impaired liver function may have elevated plasma concentrations
of felodipine and may respond to lower doses of felodipine extended-release
tablets; therefore, a starting dose of 2.5 mg once a day is recommended.
These patients should have their blood pressure monitored closely during
dosage adjustment of felodipine extended-release tablets. (See CLINICAL
PHARMACOLOGY and DOSAGE AND ADMINISTRATION.)
Peripheral Edema
Peripheral edema, generally mild and not associated with generalized fluid
retention, was the most common adverse event in the clinical trials. The
incidence of peripheral edema was both dose and age dependent. Frequency
of peripheral edema ranged from about 10% in patients under 50 years of
age taking 5 mg daily to about 30% in those over 60 years of age taking 20
mg daily. This adverse effect generally occurs within 2 to 3 weeks of the
initiation of treatment.Information for Patients
Patients should be instructed to take felodipine extended-release tablets
whole and not to crush or chew the tablets. They should be told that mild
gingival hyperplasia (gum swelling) has been reported. Good dental hygiene
decreases its incidence and severity.
NOTE: As with many other drugs, certain advice to patients being treated
with felodipine extended-release tablets is warranted. This information is
intended to aid in the safe and effective use of this medication. It is not a
disclosure of all possible adverse or intended effects.Drug Interactions
CYP3A4 Inhibitors
Felodipine is metabolized by CYP3A4. Coadministration of CYP3A4 inhibitors
(e.g., ketoconazole, itraconazole, erythromycin, grapefruit juice, cimetidine)
with felodipine may lead to several-fold increases in the plasma levels of
felodipine, either due to an increase in bioavailability or due to a decrease in
metabolism. These increases in concentration may lead to increased effects,
(lower blood pressure and increased heart rate). These effects have been
observed with coadministration of itraconazole (a potent CYP3A4 inhibitor).
Caution should be used when CYP3A4 inhibitors are coadministered with
felodipine. A conservative approach to dosing felodipine should be taken. The
following specific interactions have been reported:
Itraconazole
Coadministration of another extended-release formulation of felodipine with
itraconazole resulted in approximately 8-fold increase in the AUC, more
than 6-fold increase in the Cmax, and 2-fold prolongation in the half-life of
felodipine.
Erythromycin
Coadministration of felodipine extended-release tablets with erythromycin
resulted in approximately 2.5-fold increase in the AUC and Cmax, and about
2-fold prolongation in the half-life of felodipine.
Grapefruit Juice
Coadministration of felodipine extended-release tablets with grapefruit
juice resulted in more than 2-fold increase in the AUC and Cmax, but no
prolongation in the half-life of felodipine.
Cimetidine
Coadministration of felodipine with cimetidine (a non-specific CYP-450 inhibitor)
resulted in an increase of approximately 50% in the AUC and the Cmax, of
felodipine.
Beta-Blocking Agents
A pharmacokinetic study of felodipine in conjunction with metoprolol demonstrated
no significant effects on the pharmacokinetics of felodipine. The AUC and
Cmax of metoprolol, however, were increased approximately 31 and 38%,
respectively. In controlled clinical trials, however, beta-blockers including
metoprolol were concurrently administered with felodipine and were well
tolerated.
Digoxin
When given concomitantly with felodipine extended-release tablets the
pharmacokinetics of digoxin in patients with heart failure were not significantly
altered.
Anticonvulsants
In a pharmacokinetic study, maximum plasma concentrations of felodipine
were considerably lower in epileptic patients on long-term anticonvulsant
therapy (e.g. phenytoin, carbamazepine, or phenobarbital) than in healthy
volunteers. In such patients, the mean area under the felodipine plasma
concentration-time curve was also reduced to approximately 6% of that
observed in healthy volunteers. Since a clinically significant interaction may
be anticipated, alternative antihypertensive therapy should be considered in
these patients.
Tacrolimus
Felodipine may increase the blood concentration of tacrolimus. When given
concomitantly with felodipine, the tacrolimus blood concentration should be
followed and the tacrolimus dose may need to be adjusted.
Other Concomitant Therapy
In healthy subjects there were no clinically significant interactions when
felodipine was given concomitantly with indomethacin or spironolactone.
Interaction with Food
See CLINICAL PHARMACOLOGY: Pharmacokinetics and Metabolism.Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2 year carcinogenicity study in rats fed felodipine at doses of 7.7, 23.1
or 69.3 mg/kg/day (up to 61 times** the maximum recommended human dose
on a mg/m2 basis), a dose related increase in the incidence of benign
interstitial cell tumors of the testes (Leydig cell tumors) was observed in
treated male rats. These tumors were not observed in a similar study in mice
at doses up to 138.6 mg/kg/day (61 times** the maximum recommended
human dose on a mg/m2 basis). Felodipine, at the doses employed in the 2
year rat study, has been shown to lower testicular testosterone and to produce
a corresponding increase in serum luteinizing hormone in rats. The Leydig
cell tumor development is possibly secondary to these hormonal effects
which have not been observed in man.
In this same rat study a dose related increase in the incidence of focal
squamous cell hyperplasia compared to control was observed in the esophageal
groove of male and female rats in all dose groups. No other drug-related
esophageal or gastric pathology was observed in the rats or with chronic
administration in mice and dogs. The latter species, like man, has no anatomical
structure comparable to the esophageal groove.
Felodipine was not carcinogenic when fed to mice at doses up to 138.6 mg/kg/day
(61 times** the maximum recommended human dose on a mg/m2 basis) for
periods of up to 80 weeks in males and 99 weeks in females.
Felodipine did not display any mutagenic activity in vitro in the Ames
microbial mutagenicity test or in the mouse lymphoma forward mutation
assay. No clastogenic potential was seen in vivo in the mouse micronucleus
test at oral doses up to 2500 mg/kg (1,100 times** the maximum recommended
human dose on a mg/m2 basis) or in vitro in a human lymphocyte chromosome
aberration assay.
A fertility study in which male and female rats were administered doses of
3.8, 9.6 or 26.9 mg/kg/day (up to 24 times** the maximum recommended
human dose on a mg/m2 basis) showed no significant effect of felodipine on
reproductive performance.
**Based on patient weight of 50 kgPregnancy
Teratogenic Effects. Pregnancy Category C
Studies in pregnant rabbits administered doses of 0.46, 1.2, 2.3 and 4.6 mg/kg/day
(from 0.8 to 8 times** the maximum recommended human dose on a mg/m2
basis) showed digital anomalies consisting of reduction in size and degree
of ossification of the terminal phalanges in the fetuses. The frequency and
severity of the changes appeared dose related and were noted even at the
lowest dose. These changes have been shown to occur with other members
of the dihydropyridine class and are possibly a result of compromised uterine
blood flow. Similar fetal anomalies were not observed in rats given felodipine.
In a teratology study in cynomolgus monkeys, no reduction in the size of the
terminal phalanges was observed, but an abnormal position of the distal
phalanges was noted in about 40% of the fetuses.
Nonteratogenic Effects
A prolongation of parturition with difficult labor and an increased frequency
of fetal and early postnatal deaths were observed in rats administered doses
of 9.6 mg/kg/day (8 times** the maximum human dose on a mg/m2 basis) and
above.
**Based on patient weight of 50 kg
Significant enlargement of the mammary glands, in excess of the normal
enlargement for pregnant rabbits, was found with doses greater than or equal
to 1.2 mg/kg/day (2.1 times the maximum human dose on a mg/m2 basis).
This effect occurred only in pregnant rabbits and regressed during lactation.
Similar changes in the mammary glands were not observed in rats or
monkeys.
There are no adequate and well controlled studies in pregnant women. If
felodipine extended-release tablet is used during pregnancy, or if the
patient becomes pregnant while taking this drug, she should be apprised
of the potential hazard to the fetus, possible digital anomalies of the infant,
and the potential effects of felodipine on labor and delivery and on the
mammary glands of pregnant females.Nursing Mothers
It is not known whether this drug is secreted in human milk and because of
the potential for serious adverse reactions from felodipine in the infant, a
decision should be made whether to discontinue nursing or to discontinue
the drug, taking into account the importance of the drug to the mother.Geriatric Use
Clinical studies of felodipine did not include sufficient numbers of subjects
aged 65 and over to determine whether they respond differently from younger
subjects. Other reported clinical experience has not identified differences in
responses between the elderly and younger patients. Pharmacokinetics,
however, indicate that the availability of felodipine is increased in older
patients (see CLINICAL PHARMACOLOGY: Geriatric Use). In general, dose
selection for an elderly patient should be cautious, usually starting at the low
end of the dosing range, reflecting the greater frequency of decreased
hepatic, renal, or cardiac function, and of concomitant disease or other drug
therapy. -
ADVERSE REACTIONS
In controlled studies in the United States and overseas, approximately 3,000
patients were treated with felodipine as either the extended-release or the
immediate-release formulation.
The most common clinical adverse events reported with felodipine extendedrelease
tablets administered as monotherapy at the recommended dosage
range of 2.5 mg to 10 mg once a day were peripheral edema and headache.
Peripheral edema was generally mild, but it was age and dose related and
resulted in discontinuation of therapy in about 3% of the enrolled patients.
Discontinuation of therapy due to any clinical adverse event occurred in
about 6% of the patients receiving felodipine extended-release tablets,principally for peripheral edema, headache, or flushing.
Adverse events that occurred with an incidence of 1.5% or greater at any of
the recommended doses of 2.5 mg to 10 mg once a day (felodipine extendedrelease
tablets, N = 861; Placebo, N = 334), without regard to causality, are
compared to placebo and are listed by dose in the table below. These events are
reported from controlled clinical trials with patients who were randomized to
a fixed dose of felodipine extended-release tablets or titrated from an initial
dose of 2.5 mg or 5 mg once a day. A dose of 20 mg once a day has been
evaluated in some clinical studies. Although the antihypertensive effect of
felodipine extended-release tablets is increased at 20 mg once a day,
there is a disproportionate increase in adverse events, especially those
associated with vasodilatory effects (see DOSAGE AND ADMINISTRATION).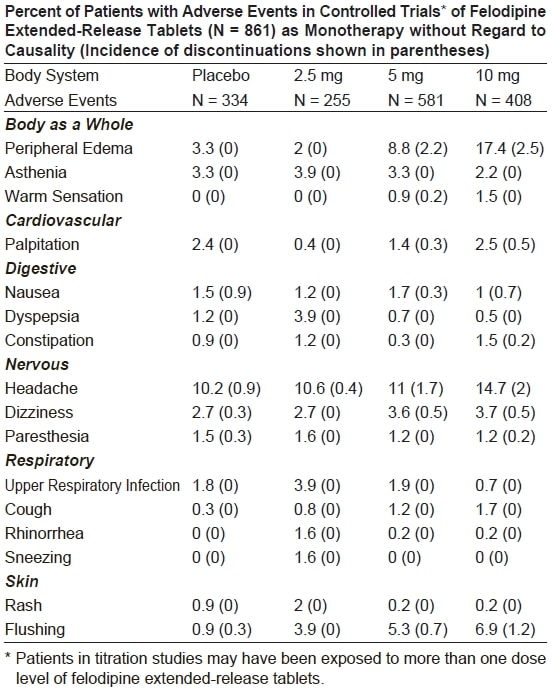
Adverse events that occurred in 0.5% up to 1.5% of patients who received
felodipine extended-release tablets in all controlled clinical trials at the
recommended dosage range of 2.5 mg to 10 mg once a day, and serious
adverse events that occurred at a lower rate, or events reported during
marketing experience (those lower rate events are in italics) are listed
below. These events are listed in order of decreasing severity within each
category, and the relationship of these events to administration of felodipine
extended-release tablets is uncertain:
Body as a Whole:Chest pain, facial edema, flu-like illness
Cardiovascular:Myocardial infarction, hypotension, syncope, angina pectoris,
arrhythmia, tachycardia, premature beats
Digestive:Abdominal pain, diarrhea, vomiting, dry mouth, flatulence, acid
regurgitation
Endocrine:Gynecomastia
Hematologic:Anemia
Metabolic:ALT (SGPT) increased
Musculoskeletal:Arthralgia, back pain, leg pain, foot pain, muscle cramps,
myalgia, arm pain, knee pain, hip pain
Nervous/Psychiatric:Insomnia, depression, anxiety disorders, irritability,
nervousness, somnolence, decreased libido
Respiratory:Dyspnea, pharyngitis, bronchitis, influenza, sinusitis, epistaxis,
respiratory infection
Skin:Angioedema, contusion, erythema, urticaria, leukocytoclastic vasculitis
Special Senses:Visual disturbances
Urogenital:Impotence, urinary frequency, urinary urgency, dysuria, polyuria.
Gingival Hyperplasia:Gingival hyperplasia, usually mild, occurred in < 0.5%
of patients in controlled studies. This condition may be avoided or may regress
with improved dental hygiene. (See PRECAUTIONS: Information for Patients.)
Clinical Laboratory Test Findings
Serum Electrolytes
No significant effects on serum electrolytes were observed during short- and
long-term therapy (see CLINICAL PHARMACOLOGY: Renal/Endocrine
Effects).
Serum Glucose
No significant effects on fasting serum glucose were observed in patients
treated with felodipine extended-release tablets in the U.S. controlled study.
Liver Enzymes
One of two episodes of elevated serum transaminases decreased once drug
was discontinued in clinical studies; no follow-up was available for the other
patient. -
OVERDOSAGE
Oral doses of 240 mg/kg and 264 mg/kg in male and female mice, respectively,
and 2390 mg/kg and 2250 mg/kg in male and female rats, respectively,
caused significant lethality.
In a suicide attempt, one patient took 150 mg felodipine together with 15 tablets
each of atenolol and spironolactone and 20 tablets of nitrazepam. The patient's
blood pressure and heart rate were normal on admission to hospital; he
subsequently recovered without significant sequelae.
Overdosage might be expected to cause excessive peripheral vasodilation
with marked hypotension and possibly bradycardia.
If severe hypotension occurs, symptomatic treatment should be instituted.
The patient should be placed supine with the legs elevated. The administration
of intravenous fluids may be useful to treat hypotension due to overdosage
with calcium antagonists. In case of accompanying bradycardia, atropine
(0.5 mg to 1 mg) should be administered intravenously. Sympathomimetic
drugs may also be given if the physician feels they are warranted.
It has not been established whether felodipine can be removed from the
circulation by hemodialysis.
To obtain up-to-date information about the treatment of overdose, consult
your Regional Poison-Control Center. Telephone numbers of certified poisoncontrol
centers are listed in the Physicians' Desk Reference (PDR). In managing
overdose, consider the possibilities of multiple-drug overdoses, drug-drug
interactions, and unusual drug kinetics in your patient. -
DOSAGE AND ADMINISTRATION
The recommended starting dose is 5 mg once a day. Depending on the patient's
response, the dosage can be decreased to 2.5 mg or increased to 10 mg
once a day. These adjustments should occur generally at intervals of not less
than 2 weeks. The recommended dosage range is 2.5 mg to 10 mg once
daily. In clinical trials, doses above 10 mg daily showed an increased blood
pressure response but a large increase in the rate of peripheral edema and
other vasodilatory adverse events (see ADVERSE REACTIONS). Modification
of the recommended dosage is usually not required in patients with renal
impairment.
Felodipine extended-release tablets should regularly be taken either without
food or with a light meal (see CLINICAL PHARMACOLOGY, Pharmacokinetics
and Metabolism). Felodipine extended-release tablets should be swallowed
whole and not crushed or chewed.Geriatric Use
Patients over 65 years of age are likely to develop higher plasma concentrations
of felodipine (see CLINICAL PHARMACOLOGY). In general, dose selection
for an elderly patient should be cautious, usually starting at the low end of the
dosing range (2.5 mg daily). Elderly patients should have their blood pressure
closely monitored during any dosage adjustment.Patients with Impaired Liver Function
Patients with impaired liver function may have elevated plasma concentrations
of felodipine and may respond to lower doses of felodipine extended-release
tablets; therefore, patients should have their blood pressure monitored closely
during dosage adjustment of felodipine extended-release tablets (see CLINICAL
PHARMACOLOGY). -
HOW SUPPLIED
Felodipine Extended-release Tablets, USP are available containing 2.5 mg,
5 mg or 10 mg of felodipine, USP.
The 2.5 mg tablets are white film-coated, round, unscored tablets debossed
with “YSP 211 ”on one side of the tablets. They are available as follows:
NDC 51407-087-01 bottles of 100 tablets
NDC 51407-087-05 bottles of 500 tablets
The 5 mg tablets are orange film-coated, round, unscored tablets debossed
with “YSP 210” on one side of the tablets. They are available as follows:
NDC 51407-088-01 bottles of 100 tablets
NDC 51407-088-05 bottles of 500 tablets
The 10 mg tablets are red film-coated, round, unscored tablets debossed with
“YSP 051” on one side of the tablets. They are available as follows:
NDC 51407-089-01 bottles of 100 tablets
NDC 51407-089-05 bottles of 500 tablets
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light.
Dispense in a tight, light-resistant container as defined in the USP using a
child-resistant closure.
Manufactured by:
Yung Shin Pharmaceutical Ind. Co., Ltd.
Tachia, Taichung 43769, TAIWAN
Distributed by:
Carlsbad Technology, Inc.
5922 Farnsworth Court, Carlsbad, CA 92008, USA
Revised: 03/2019Marketed by:
GSMS, Inc.
Camarillo, CA 93012 USA
- Principal Display Panel – Bottle Label NDC 51407-087-01 Felodipine Tablets Rx Only 100 Tablets
- Principal Display Panel – Bottle Label NDC 51407-088-01 Felodipine Tablets Rx Only 100 Tablets
- Principal Display Panel – Bottle Label NDC 51407-089-01 Felodipine Tablets Rx Only 100 Tablets
-
INGREDIENTS AND APPEARANCE
FELODIPINE
felodipine tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51407-089(NDC:61442-433) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FELODIPINE (UNII: OL961R6O2C) (FELODIPINE - UNII:OL961R6O2C) FELODIPINE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE 2208 (100 MPA.S) (UNII: B1QE5P712K) MAGNESIUM STEARATE (UNII: 70097M6I30) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) POLYETHYLENE GLYCOL 1000 (UNII: U076Q6Q621) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red Score no score Shape ROUND Size 11mm Flavor Imprint Code YSP;051 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51407-089-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/13/2020 2 NDC:51407-089-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 03/13/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204800 04/29/2019 FELODIPINE
felodipine tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51407-088(NDC:61442-432) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FELODIPINE (UNII: OL961R6O2C) (FELODIPINE - UNII:OL961R6O2C) FELODIPINE 5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE 2208 (100 MPA.S) (UNII: B1QE5P712K) MAGNESIUM STEARATE (UNII: 70097M6I30) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) POLYETHYLENE GLYCOL 1000 (UNII: U076Q6Q621) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange Score no score Shape ROUND Size 11mm Flavor Imprint Code YSP;210 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51407-088-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/13/2020 2 NDC:51407-088-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 03/13/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204800 04/29/2019 FELODIPINE
felodipine tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51407-087(NDC:61442-431) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FELODIPINE (UNII: OL961R6O2C) (FELODIPINE - UNII:OL961R6O2C) FELODIPINE 2.5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE 2208 (100 MPA.S) (UNII: B1QE5P712K) MAGNESIUM STEARATE (UNII: 70097M6I30) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) POLYETHYLENE GLYCOL 1000 (UNII: U076Q6Q621) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape ROUND Size 11mm Flavor Imprint Code YSP;211 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51407-087-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/13/2020 10/31/2023 2 NDC:51407-087-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 03/13/2020 02/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204800 04/29/2019 02/29/2024 Labeler - Golden State Medical Supply, Inc. (603184490) Establishment Name Address ID/FEI Business Operations Golden State Medical Supply, Inc. 603184490 repack(51407-087, 51407-088, 51407-089) , relabel(51407-087, 51407-088, 51407-089)






