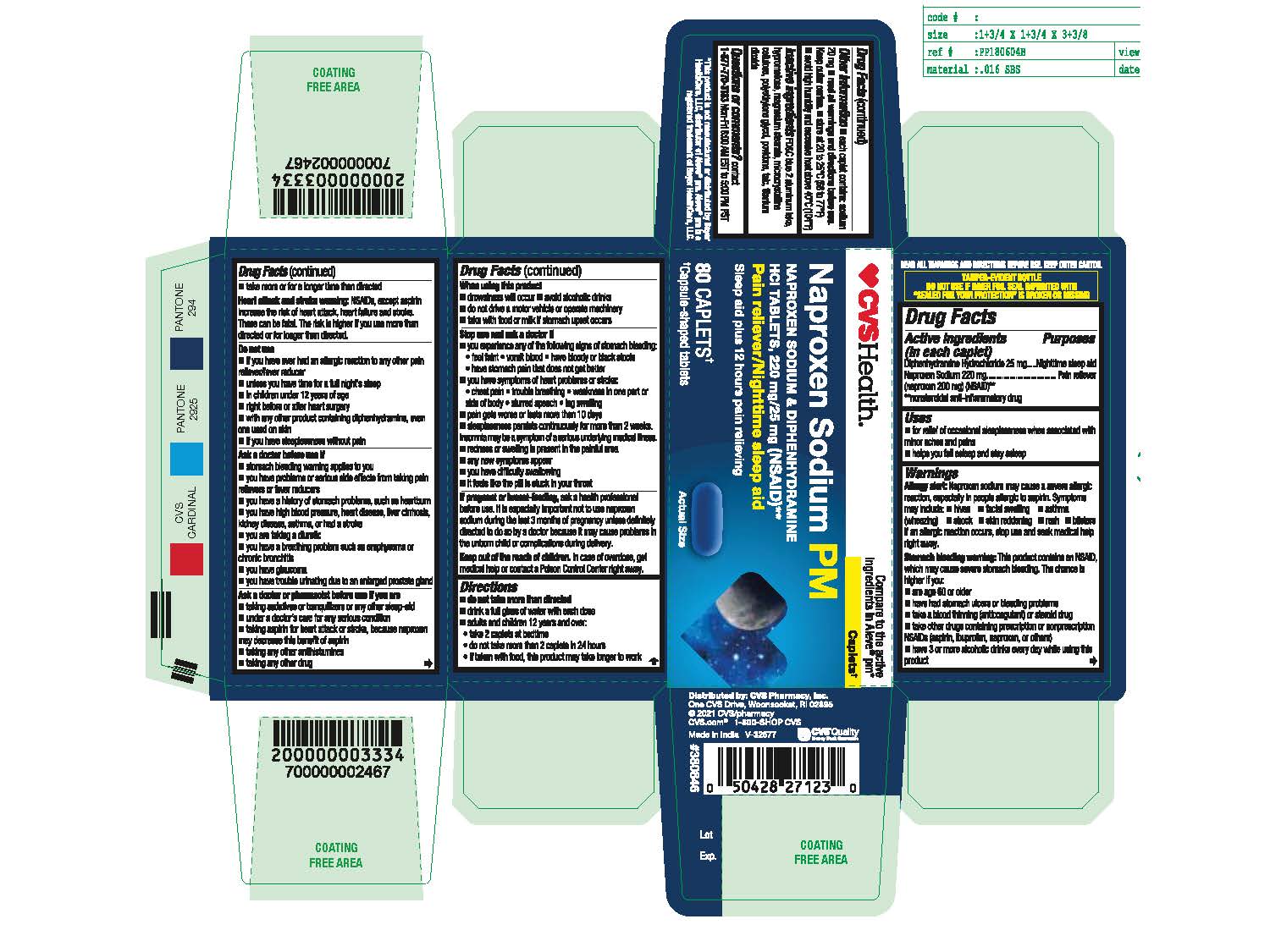
Label: NAPROXEN SODIUM DIPHENHYDRAMINE HCL- naproxen sodium diphenhydramine hcl tablet, film coated
- NDC Code(s): 69842-328-02, 69842-328-08
- Packager: CVS PHARMACY, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each caplet)
- Purposes
- Uses
- Allergy alert
-
Stomach bleeding warning
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
• are age 60 or older
• have had stomach ulcers or bleeding problems
• take a blood thinning (anticoagulant) or steroid drug
• take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen or others)
• have 3 or more alcoholic drinks every day while using this product
• take more or for a longer time than directed - Heart attack and stroke warning
-
Do not use
• if you have ever had an allergic reaction to any other pain reliever/fever reducer
• unless you have time for a full night’s sleep
• in children under 12 years of age
• right before or after heart surgery
• with any other product containing diphenhydramine, even one used on skin
• if you have sleeplessness without pain -
Ask a doctor before use if
• stomach bleeding warning applies to you
• you have problems or serious side effects from taking pain relievers or fever reducers
• you have a history of stomach problems, such as heartburn
• you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma or had a stroke
• you are taking a diuretic
• you have a breathing problem such as emphysema or chronic bronchitis
• you have glaucoma
• you have trouble urinating due to an enlarged prostate gland - Ask a doctor or pharmacist before use if you are
- When using this product
-
Stop use and ask a doctor if
• you experience any of the following signs of stomach bleeding: • feel faint • vomit blood • have bloody or black stools
• have stomach pain that does not get better
• you have symptoms heart problems or stroke:
• chest pain • trouble breathing • weakness in one part or side of body • slurred speech • leg swelling• pain gets worse or lasts more than 10 days
• sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
• redness or swelling is present in the painful area
• any new symptoms appear
• you have difficulty swallowing
• it feels like the pill is stuck in your throat - If pregnant or breast-feeding,
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Naproxen Sodium & Diphenhydramine HCl 220mg/25mg
-
INGREDIENTS AND APPEARANCE
NAPROXEN SODIUM DIPHENHYDRAMINE HCL
naproxen sodium diphenhydramine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-328 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPROXEN SODIUM (UNII: 9TN87S3A3C) (NAPROXEN - UNII:57Y76R9ATQ) NAPROXEN SODIUM 220 mg DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 2--ALUMINUM LAKE (UNII: 4AQJ3LG584) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) TALC (UNII: 7SEV7J4R1U) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) Product Characteristics Color blue Score no score Shape OVAL (modified capsule shaped biconvex film coated tablet) Size 15mm Flavor Imprint Code G;17 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-328-02 20 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 2 NDC:69842-328-08 80 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 02/28/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213663 05/14/2021 Labeler - CVS PHARMACY, INC (062312574)