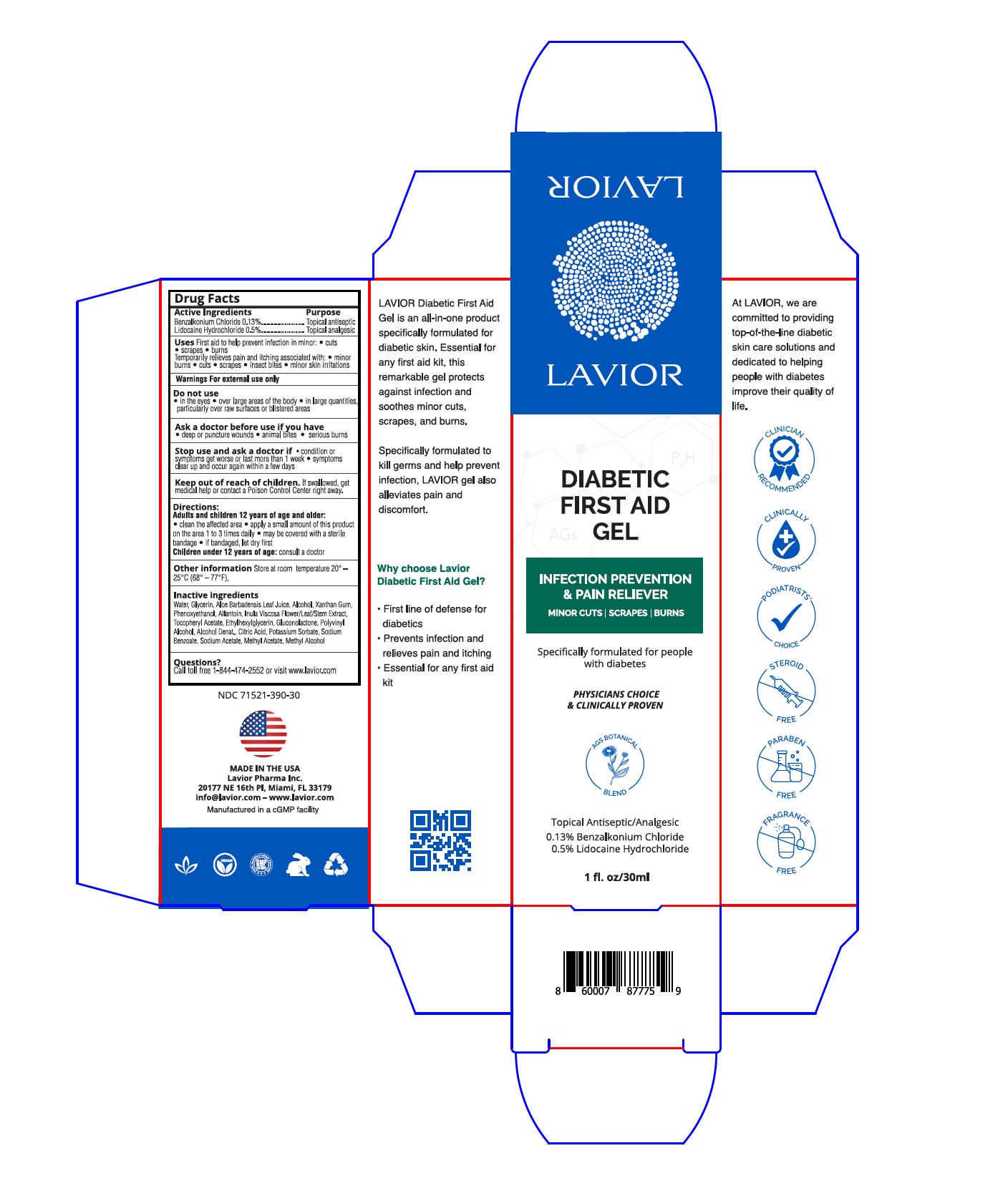
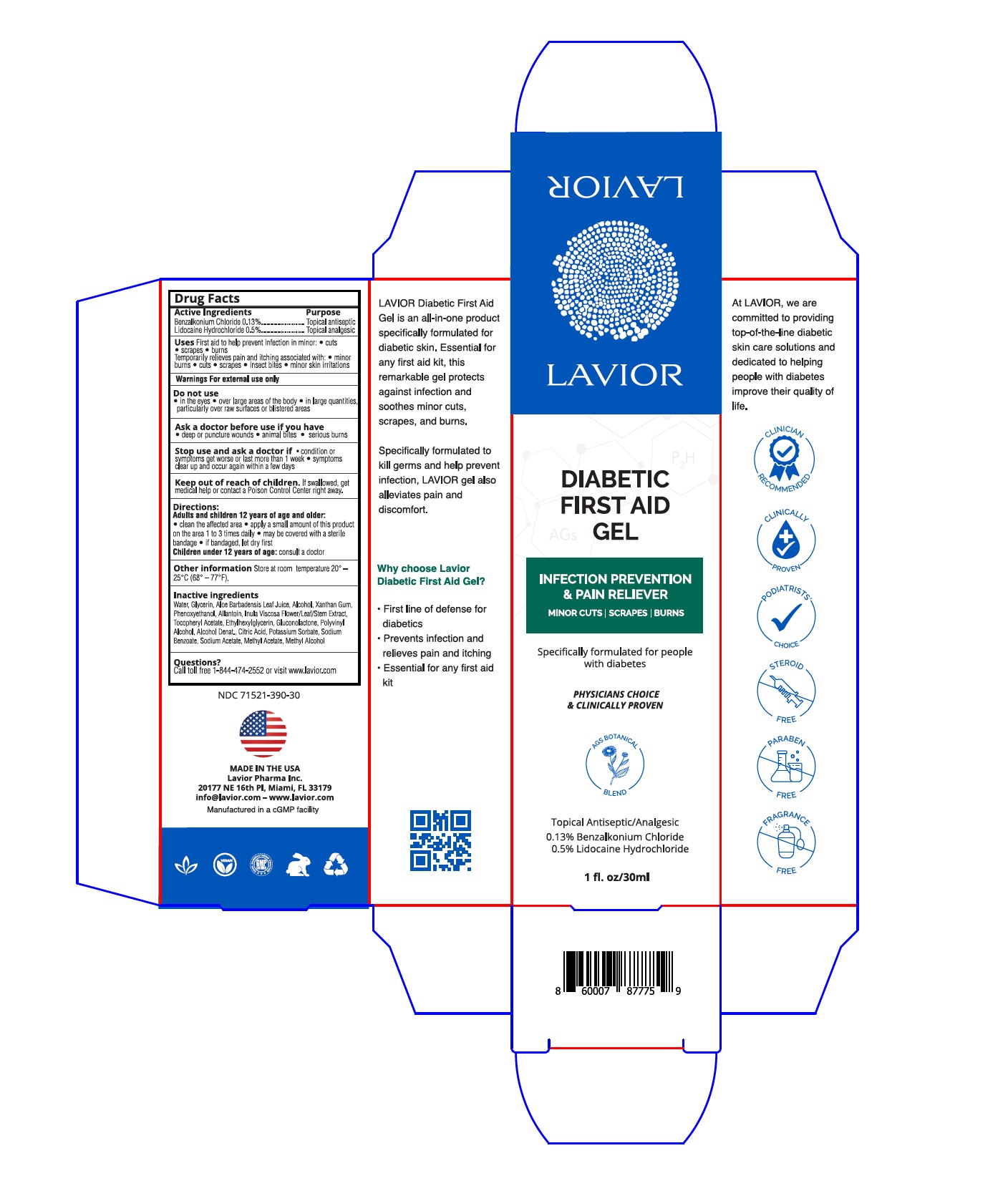
Label: DIABETIC FIRST AID GEL- benzalkonium chloride, lidocaine hydrochloride gel
- NDC Code(s): 71521-390-15, 71521-390-30, 71521-390-50
- Packager: Lavior Pharma Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- Other information
- Inactive Ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIABETIC FIRST AID GEL
benzalkonium chloride, lidocaine hydrochloride gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71521-390 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 0.5 g in 100 g BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ALCOHOL (UNII: 3K9958V90M) GLUCONOLACTONE (UNII: WQ29KQ9POT) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM BENZOATE (UNII: OJ245FE5EU) ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) DITTRICHIA VISCOSA WHOLE (UNII: 3SYW69FH88) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71521-390-50 50 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 03/31/2021 2 NDC:71521-390-15 15 g in 1 TUBE; Type 0: Not a Combination Product 03/17/2022 3 NDC:71521-390-30 30 g in 1 TUBE; Type 0: Not a Combination Product 04/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 03/31/2021 Labeler - Lavior Pharma Inc. (080685327)