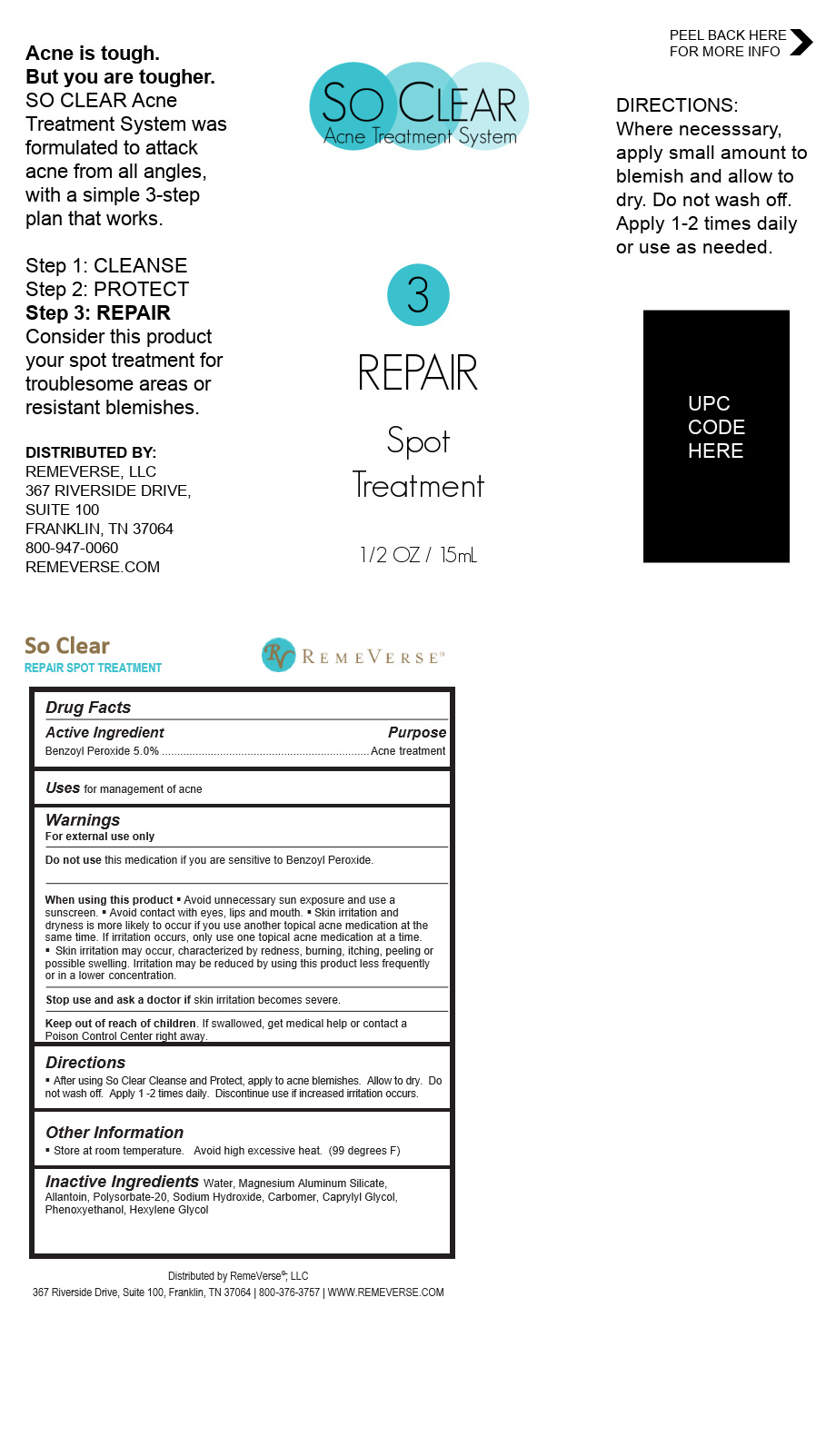
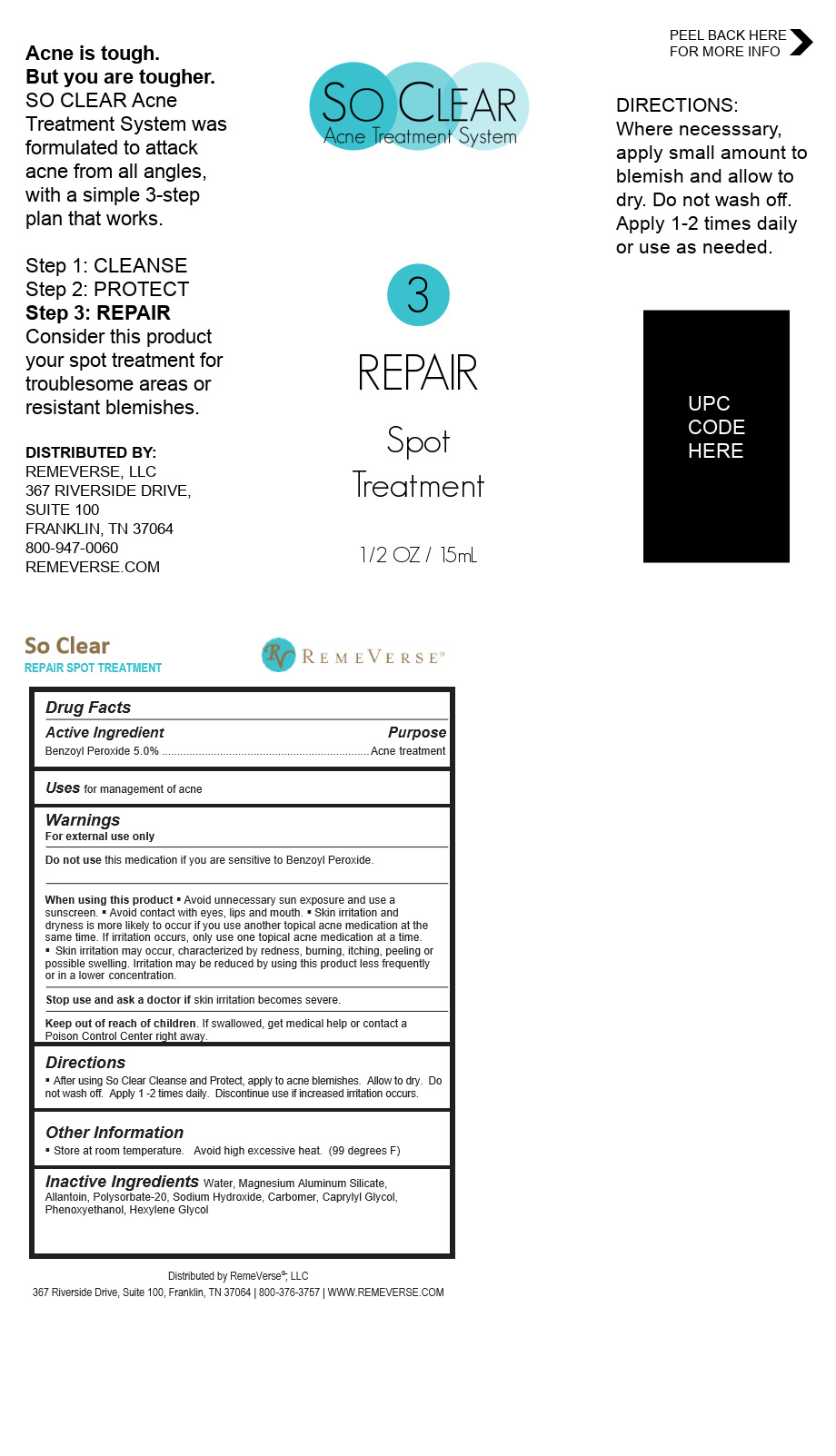
Label: SO CLEAR REPAIR SPOT TREATMENT liquid
- NDC Code(s): 70317-148-15
- Packager: RemeVerse LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- So Clear Repair Spot Treatment
- So Clear Repair Spot Treatment
- Directions
- Uses
- SPL UNCLASSIFIED SECTION
-
WARNINGS
Warnings
For external use only
Do not use this medication if you are sensitive to Benzoyl Peroxide.
When using this product § Avoid unnecessary sun exposure and use a sunscreen. § Avoid contact with eyes, lips and mouth. § Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Skin irritation may occur, characterized by redness, burning, itching, peeling or possible swelling. Irritation may be reduced by using this product less frequently or in a lower concentration.
Stop use and ask a doctor if skin irritation become severe
Keep out of the reach of children If swallowed, get medical help or contact a Poison Control Center right away.
- STOP USE
- PURPOSE
- Other Information
- Keep out of the reach of children
- So Clear Repair
-
INGREDIENTS AND APPEARANCE
SO CLEAR REPAIR SPOT TREATMENT
so clear repair spot treatment liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70317-148 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 0.7875 g in 15 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 20 (UNII: 7T1F30V5YH) HEXYLENE GLYCOL (UNII: KEH0A3F75J) CARBOMER 940 (UNII: 4Q93RCW27E) ALLANTOIN (UNII: 344S277G0Z) OCTYLPHENOXYETHANOL (UNII: 5G75D69KFO) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color Score Shape ROUND Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70317-148-15 15 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 03/31/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 03/31/2021 Labeler - RemeVerse LLC (050186540) Registrant - Lexia LLC (015552120) Establishment Name Address ID/FEI Business Operations Lexia LLC 015552120 manufacture(70317-148)