Label: DANTROLENE SODIUM capsule
- NDC Code(s): 50268-217-11, 50268-217-15
- Packager: AvPAK
- This is a repackaged label.
- Source NDC Code(s): 0115-4411
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 17, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION(25 mg, 50 mg and 100 mg) Rx only
-
BOXED WARNING
(What is this?)
Dantrolene sodium has a potential for hepatotoxicity, and should not be used in conditions other than those recommended. Symptomatic hepatitis (fatal and non-fatal) has been reported at various dose levels of the drug. The incidence reported in patients taking up to 400 mg/day is much lower than in those taking doses of 800 mg or more per day. Even sporadic short courses of these higher dose levels within a treatment regimen markedly increased the risk of serious hepatic injury. Liver dysfunction as evidenced by blood chemical abnormalities alone (liver enzyme elevations) has been observed in patients exposed to dantrolene sodium for varying periods of time. Overt hepatitis has occurred at varying intervals after initiation of therapy, but has been most frequently observed between the third and twelfth month of therapy. The risk of hepatic injury appears to be greater in females, in patients over 35 years of age, and in patients taking other medication(s) in addition to dantrolene sodium. Spontaneous reports suggest a higher proportion of hepatic events with fatal outcome in elderly patients receiving dantrolene sodium. However, the majority of these cases were complicated with confounding factors such as intercurrent illnesses and/or concomitant potentially hepatotoxic medications (see Geriatric Use subsection). Dantrolene sodium should be used only in conjunction with appropriate monitoring of hepatic function including frequent determination of SGOT or SGPT. If no observable benefit is derived from the administration of dantrolene sodium after a total of 45 days, therapy should be discontinued. The lowest possible effective dose for the individual patient should be prescribed.
Close -
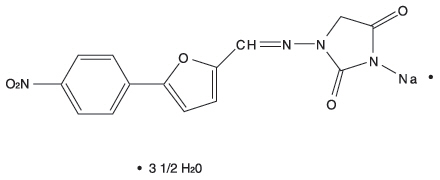
DESCRIPTIONThe chemical formula of dantrolene sodium is hydrated 1-[[[5-(4-nitrophenyl)-2-furanyl]methylene] amino]-2, 4-imidazolidinedione sodium salt. It is an orange powder, slightly soluble in water, but ...
-
CLINICAL PHARMACOLOGYIn isolated nerve-muscle preparation, dantrolene sodium has been shown to produce relaxation by affecting the contractile response of the skeletal muscle at a site beyond the myoneural junction ...
-
INDICATIONS AND USAGEIn Chronic Spasticity - Dantrolene sodium capsules are indicated in controlling the manifestations of clinical spasticity resulting from upper motor neuron disorders (e.g., spinal cord injury ...
-
CONTRAINDICATIONSActive hepatic disease, such as hepatitis and cirrhosis, is a contraindication for use of dantrolene sodium capsules. Dantrolene sodium capsules are contraindicated where spasticity is utilized to ...
-
WARNINGSIt is important to recognize that fatal and non-fatal liver disorders of an idiosyncratic or hypersensitivity type may occur with dantrolene sodium therapy. At the start of dantrolene sodium ...
-
PRECAUTIONSDantrolene sodium should be used with caution in patients with impaired pulmonary function, particularly those with obstructive pulmonary disease, and in patients with severely impaired cardiac ...
-
ADVERSE REACTIONSThe most frequently occurring side effects of dantrolene sodium have been drowsiness, dizziness, weakness, general malaise, fatigue, and diarrhea. These are generally transient, occurring early in ...
-
DRUG ABUSE AND DEPENDENCEDrug abuse and dependency potential has not been evaluated in human or animal studies.
-
OVERDOSAGESymptoms which may occur in case of overdose include, but are not limited to, muscular weakness and alterations in the state of consciousness (e.g., lethargy, coma), vomiting, diarrhea, and ...
-
DOSAGE AND ADMINISTRATIONFor Use in Chronic Spasticity - Prior to the administration of dantrolene sodium capsules, consideration should be given to the potential response to treatment. A decrease in spasticity ...
-
HOW SUPPLIEDDantrolene Sodium Capsules USP, 25 mg are rich yellow opaque bodies and light green opaque caps. Each cap and body imprinted in black with G441. They are available as follows: NDC ...
-
SPL UNCLASSIFIED SECTIONManufactured for: AvKARE - Pulaski, TN 38478 - Mfg. Rev. 10-2020-00 - AV Rev. 04/25 (M) AvPAK
-
PRINCIPAL DISPLAY PANEL - 25 mg-100ct Bottle Label

-
INGREDIENTS AND APPEARANCEProduct Information