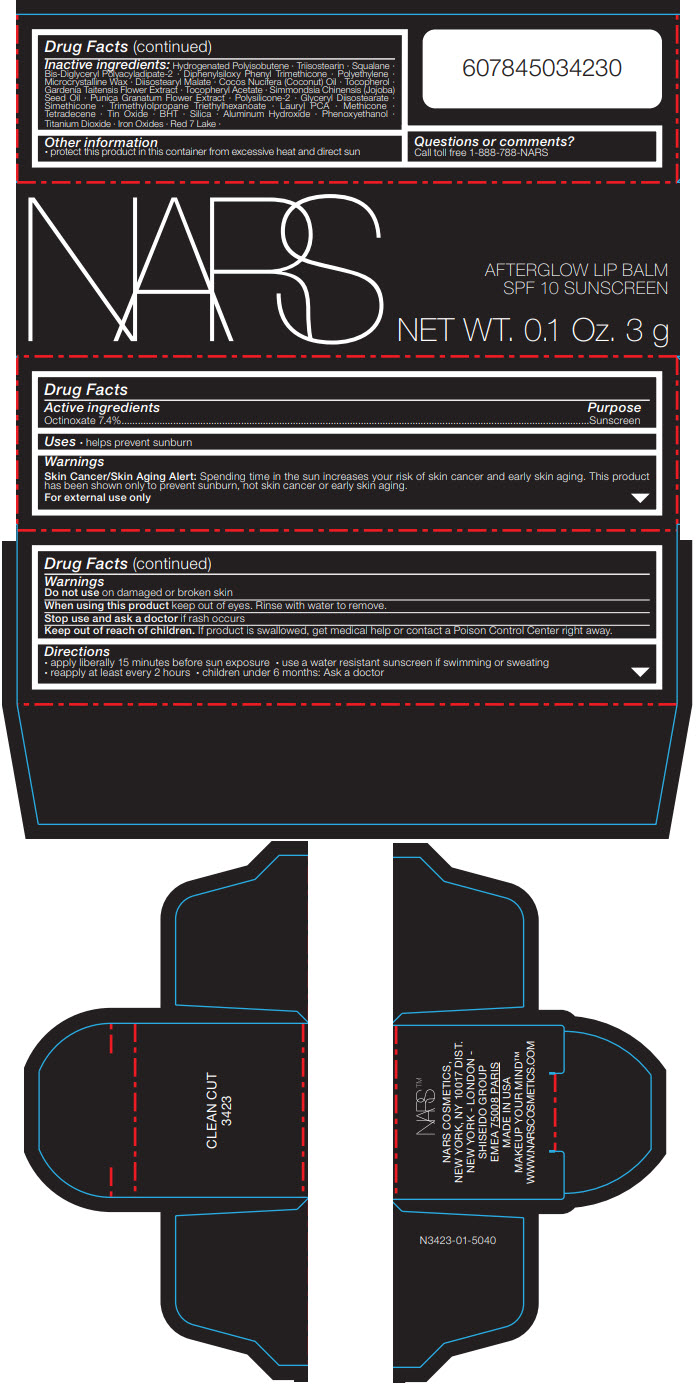
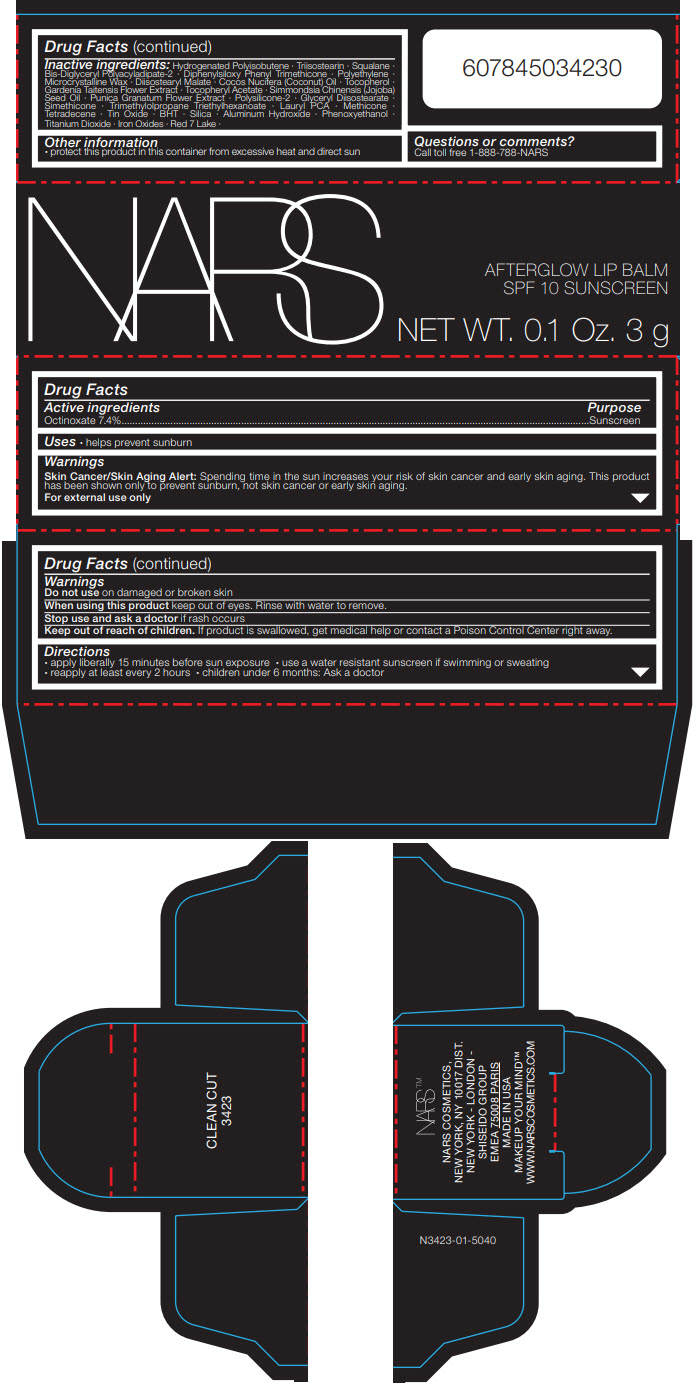
Label: NARS AFTERGLOW LIP BALM CLEAN CUT- octinoxate stick
- NDC Code(s): 13734-160-20
- Packager: NARS Cosmetics
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 9, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
Hydrogenated Polyisobutene ∙ Triisostearin ∙ Squalane ∙ Bis-Diglyceryl Polyacyladipate-2 ∙ Diphenylsiloxy Phenyl Trimethicone ∙ Polyethylene ∙ Microcrystalline Wax ∙ Diisostearyl Malate ∙ Cocos Nucifera (Coconut) Oil ∙ Tocopherol ∙ Gardenia Taitensis Flower Extract ∙ Tocopheryl Acetate ∙ Simmondsia Chinensis (Jojoba) Seed Oil ∙ Punica Granatum Flower Extract ∙ Polysilicone-2 ∙ Glyceryl Diisostearate ∙ Simethicone ∙ Trimethylolpropane Triethylhexanoate ∙ Lauryl PCA ∙ Methicone ∙ Tetradecene ∙ Tin Oxide ∙ BHT ∙ Silica ∙ Aluminum Hydroxide ∙ Phenoxyethanol ∙ Titanium Dioxide ∙ Iron Oxides ∙ Red 7 Lake ∙
- Other information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 3 g Cartridge Carton
-
INGREDIENTS AND APPEARANCE
NARS AFTERGLOW LIP BALM CLEAN CUT
octinoxate stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13734-160 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 222 mg in 3 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYISOBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) TRIISOSTEARIN (UNII: 71503RH8KG) SQUALANE (UNII: GW89575KF9) BIS-DIGLYCERYL POLYACYLADIPATE-2 (UNII: 6L246LAM9T) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) COCONUT OIL (UNII: Q9L0O73W7L) GLYCERYL DIISOSTEARATE (UNII: 68BAV42LRC) PHENOXYETHANOL (UNII: HIE492ZZ3T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GARDENIA TAITENSIS FLOWER (UNII: T0OCU8201Y) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) TRIMETHYLOLPROPANE TRIETHYLHEXANOATE (UNII: B952ZGW1IL) LAURYL PIDOLATE (UNII: 29C5O2BJYA) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) JOJOBA OIL (UNII: 724GKU717M) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) METHICONE (20 CST) (UNII: 6777U11MKT) 1-TETRADECENE (UNII: FW23481S7S) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13734-160-20 1 in 1 CARTON 02/01/2019 1 3 g in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M020 02/01/2019 Labeler - NARS Cosmetics (837363571) Establishment Name Address ID/FEI Business Operations SHISEIDO AMERICA INC. 782677132 MANUFACTURE(13734-160) , ANALYSIS(13734-160)