Label: ATENOLOL tablet
- NDC Code(s): 75834-281-10, 75834-282-10, 75834-283-10
- Packager: Nivagen Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
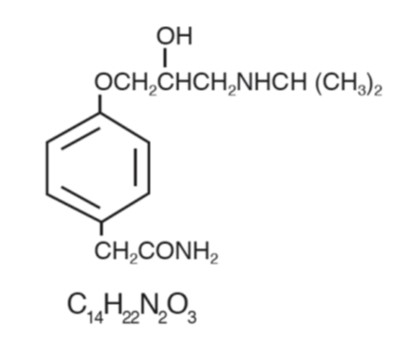
DESCRIPTIONAtenolol, a synthetic, beta1-selective (cardioselective) adrenoreceptor blocking agent, may be chemically described as benzeneacetamide, 4-[2’-hydroxy-3’-[(1-methylethyl)amino]propoxy]-. The ...
-
CLINICAL PHARMACOLOGYAtenolol is a beta1-selective (cardioselective) beta-adrenergic receptor blocking agent without membrane stabilizing or intrinsic sympathomimetic (partial agonist) activities. This preferential ...
-
INDICATIONS & USAGEHypertension - Atenolol tablets, USP are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular ...
-
CONTRAINDICATIONSAtenolol tablets are contraindicated in sinus bradycardia, heart block greater than first degree, cardiogenic shock, and overt cardiac failure. (SeeWARNINGS.) Atenolol tablets are ...
-
WARNINGSCardiac Failure - Sympathetic stimulation is necessary in supporting circulatory function in congestive heart failure, and beta blockade carries the potential hazard of further depressing ...
-
PRECAUTIONSGENERAL PRECAUTIONS - Patients already on a beta-blocker must be evaluated carefully before atenolol is administered. Initial and subsequent atenolol dosages can be adjusted downward depending on ...
-
ADVERSE REACTIONSMost adverse effects have been mild and transient. The frequency estimates in the following table were derived from controlled studies in hypertensive patients in which adverse reactions were ...
-
OVERDOSAGEOverdosage with atenolol has been reported with patients surviving acute doses as high as 5 g. One death was reported in a man who may have taken as much as 10 g acutely. The predominant ...
-
DOSAGE & ADMINISTRATIONHypertension - The initial dose of atenolol is 50 mg given as one tablet a day either alone or added to diuretic therapy. The full effect of this dose will usually be seen within one to two weeks ...
-
HOW SUPPLIEDAtenolol tablets, USP for oral administration, are supplied as: Tablets of 25 mg atenolol are round, white, flat beveled edge tablet, debossed 25 on one side and L13 on the reverse side, and ...
-
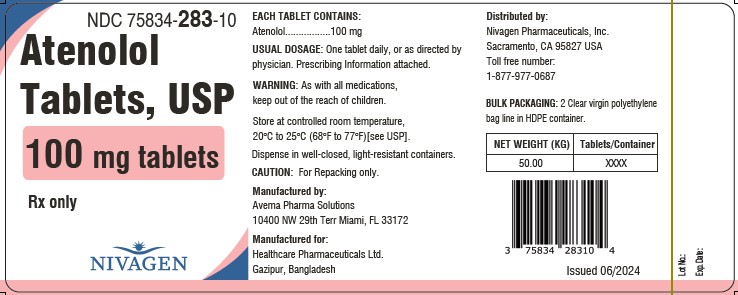
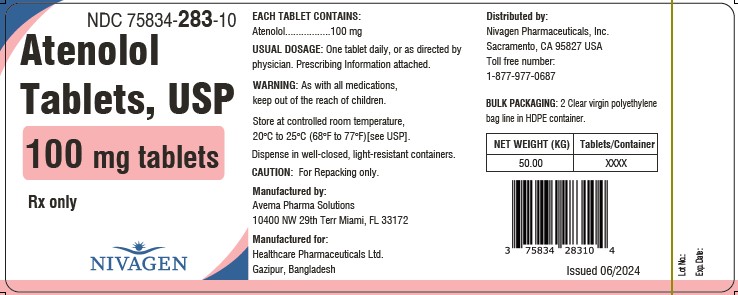
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 75834-281-10 - Rx Only - Atenolol tablets, USP - 25 mg Tablets - 50 Kg Tablets - NDC 75834-282-10 - Rx Only - Atenolol tablets, USP - 50 mg Tablets - 50 Kg Tablets - NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information