Label: DERMELEVE- aluminum acetate cream
- NDC Code(s): 81507-002-01, 81507-002-02, 81507-002-03, 81507-002-04, view more
- Packager: Advanced Derm Solutions LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 29, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
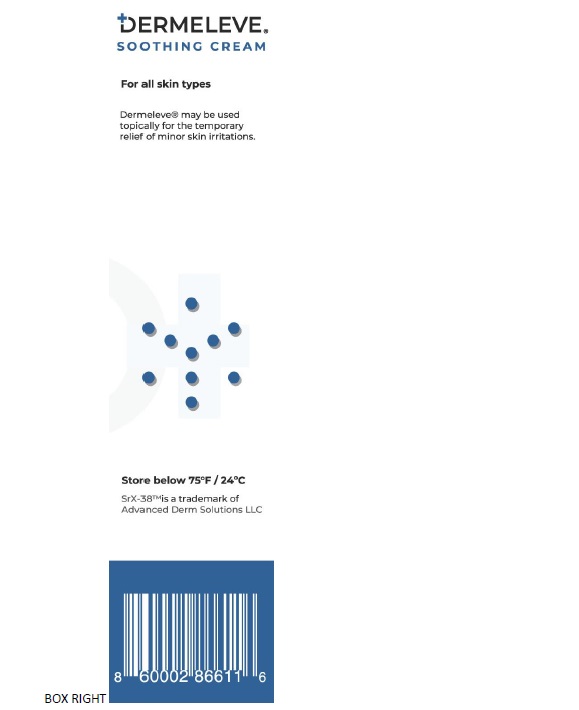
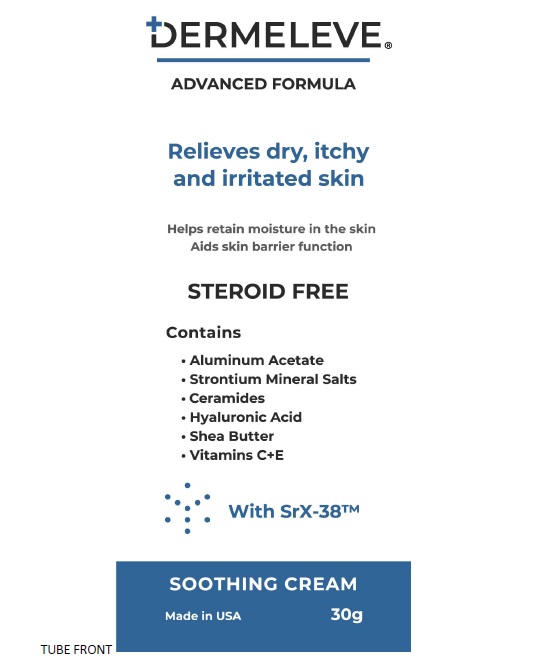
Active IngredientAluminum acetate (0.2%)
-
PurposeAstringent
-
Uses• Insects Bites - • Minor Burn - • Athlete's foot • Poison lvy • Poison oak - • Poison sumac • Rashes cause by soaps, detergents, cosmetic or jewelry
-
Warnings■ For external use only. ■ Avoidcontact with eyes. ■ Do not applyto open wounds. ■ STOP USEand ask a doctor if condition worsens - or symptoms persist for more ...
- KEEP OUT OF REACH OF CHILDREN
-
DirectionsAdults and children two years of age and older, apply to affected area as needed or as directed by a doctor. Consult a doctor for children under the age of two.
-
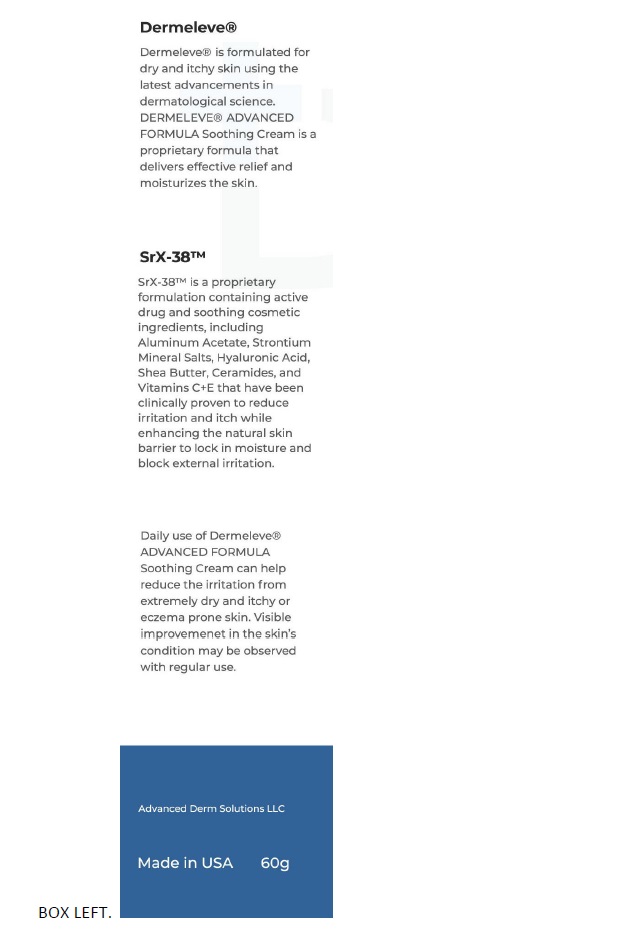
Inactive ingredients Butyrospermum Parkii (Shea) Butter, Caprylic/Capric Triglyceride, Caprylyl Glycol, Ceramide NG, Cetearyl Alcohol, Cetyl Alcohol, Citric Acid - Dimethicone, Disodium EDTA, Glycerin, Glyceryl ...
-
SPL UNCLASSIFIED SECTIONQuestions ? Visit www.dermeleve.com
-
SPL UNCLASSIFIED SECTIONManufactured for Advanced Derm Solutions LLC - Ocala, FL 34482 | dermeleve.com - U.S. Patents: 5,716,625; 5,804,203; 6,139,850; 7,404,967
-
Product label
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information