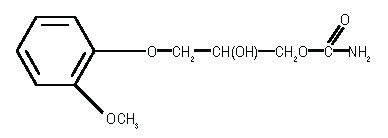Label: METHOCARBAMOL injection
- NDC Code(s): 70436-149-33, 70436-149-55
- Packager: Slate Run Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Rx only
-
DESCRIPTION
Methocarbamol injection, USP, a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant with sedative and musculoskeletal relaxant properties. It is a sterile ...
-
CLINICAL PHARMACOLOGYThe mechanism of action of methocarbamol in humans has not been established, but may be due to general CNS depression. It has no direct action on the contractile mechanism of striated muscle, the ...
-
INDICATIONS AND USAGEThe injectable form of methocarbamol is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal ...
-
CONTRAINDICATIONSMethocarbamol Injection should not be administered to patients with known or suspected renal pathology. This caution is necessary because of the presence of polyethylene glycol 300 in the ...
-
WARNINGSSince methocarbamol may possess a general CNS depressant effect, patients receiving Methocarbamol Injection should be cautioned about combined effects with alcohol and other CNS depressants. Safe ...
-
PRECAUTIONSGeneral - As with other agents administered either intravenously or intramuscularly, careful supervision of dose and rate of injection should be observed. Rate of injection should not exceed 3 mL ...
-
ADVERSE REACTIONSThe following adverse reactions have been reported coincident with the administration of methocarbamol. Some events may have been due to an overly rapid rate of intravenous injection. Body as a ...
-
OVERDOSAGELimited information is available on the acute toxicity of methocarbamol. Overdose of methocarbamol is frequently in conjunction with alcohol or other CNS depressants and includes the following ...
-
DOSAGE AND ADMINISTRATIONFor Intravenous and Intramuscular Use Only.Total adult dosage should not exceed 30 mL (3 vials) a day for more than 3 consecutive days except in the treatment of tetanus. If the condition ...
-
HOW SUPPLIEDMethocarbamol Injection, USP, (100 mg/mL) is supplied in 10 mL single-dose vials (NDC 70436-149-33), in cartons of 25 vials (NDC 70436-149-55). Store at 20° to 25°C (68° to 77°F); excursions ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELMethocarbamol Injection, USP, 1,000 mg/10 mL (100 mg/mL), 10 mL Single-Dose Vial - NDC 70436-149-33
-
INGREDIENTS AND APPEARANCEProduct Information