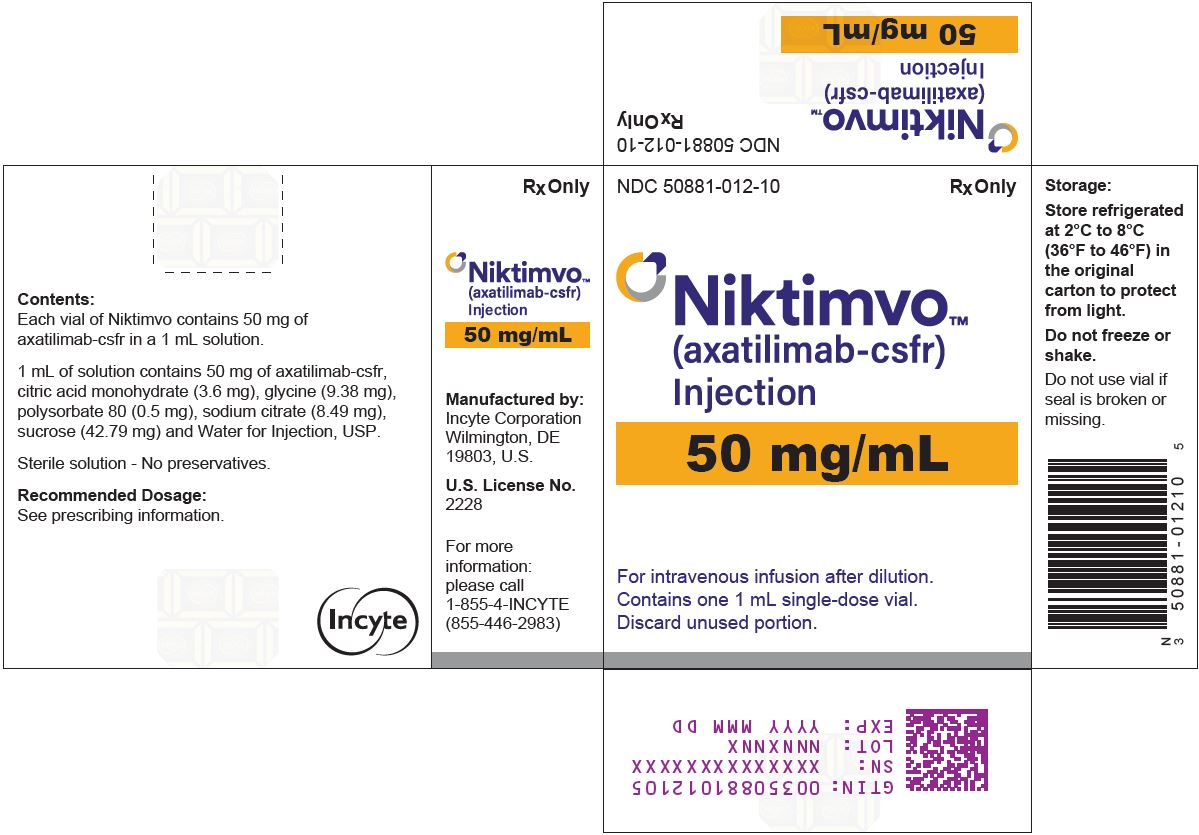
Label: NIKTIMVO- axatilimab-csfr injection
- NDC Code(s): 50881-012-10, 50881-023-11, 50881-034-12
- Packager: Incyte Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NIKTIMVO safely and effectively. See full prescribing information for NIKTIMVO. NIKTIMVO™ (axatilimab-csfr) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENIKTIMVO is indicated for the treatment of chronic graft-versus-host disease (cGVHD) after failure of at least two prior lines of systemic therapy in adult and pediatric patients weighing at least ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - For patients weighing at least 40 kg, administer NIKTIMVO 0.3 mg/kg, up to a maximum dose of 35 mg, as an intravenous infusion over 30 minutes every 2 weeks until ...
-
3 DOSAGE FORMS AND STRENGTHSNIKTIMVO injection is a slightly opalescent, pale brownish yellow solution available as: 9 mg/0.18 mL in a single-dose vial. 22 mg/0.44 mL in a single-dose vial. 50 mg/mL in a single-dose ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Infusion-Related Reactions - NIKTIMVO can cause infusion-related reactions. Infusion-related reactions, including hypersensitivity reactions, occurred in 18% of patients who received NIKTIMVO ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in the labeling. Infusion-Related Reactions [see Warnings and Precautions (5.1)] 6.1 Clinical Trial Experience - Because clinical trials ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action, NIKTIMVO may cause fetal harm when administered to pregnant women [see Clinical Pharmacology (12.1)]. There are no available data ...
-
11 DESCRIPTIONAxatilimab-csfr is a CSF-1R–blocking antibody. Axatilimab-csfr is a humanized IgG4 (kappa light chain) monoclonal antibody produced in Chinese hamster ovary cells. Axatilimab-csfr has an ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Axatilimab-csfr is a monoclonal antibody that binds to colony stimulating factor-1 receptors (CSF-1R) expressed on monocytes and macrophages. Blocking CSF-1R with ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been performed to assess the potential of axatilimab-csfr for carcinogenicity or genotoxicity. Dedicated fertility ...
-
14 CLINICAL STUDIESThe efficacy of NIKTIMVO was evaluated in AGAVE-201 (NCT04710576), a randomized, open-label, multicenter study in adult and pediatric patients with recurrent or refractory cGVHD who had received ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNIKTIMVO (axatilimab-csfr) injection is a slightly opalescent, pale brownish yellow solution. It is supplied in a carton containing one single-dose vial either as: 9 mg/0.18 mL (NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Infusion-Related Reactions - Advise patients and caregivers that reactions related to the infusion may ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - NIKTIMVO™ (nik tim voe) (axatilimab-csfr) injection, for intravenous use - What is NIKTIMVO? NIKTIMVO is a prescription medicine used to treat adults and ...
-
NIKTIMVO 50 MG/ML CARTONNDC 50881-012-10 - Rx Only - NIKTIMVO™ (axatilimab-csfr) Injection - 50 mg/mL - For intravenous infusion after dilution. Contains one 1 mL single-dose vial. Discard unused portion.
-
NIKTIMVO 9 MG/0.18ML CARTONNDC 50881-034-12 - Rx Only - NIKTIMVO™ (axatilimab-csfr) Injection - 9 mg/0.18mL - For intravenous infusion after dilution. Contains one 0.18 mL single-dose vial. Discard unused portion.
-
NIKTIMVO 22 MG/0.44ML CARTONNDC 50881-023-11 - Rx Only - NIKTIMVO™ (axatilimab-csfr) Injection - 22 mg/0.44mL - For intravenous infusion after dilution. Contains one 0.44 mL single-dose vial. Discard unused portion.
-
INGREDIENTS AND APPEARANCEProduct Information