Label: METHSCOPOLAMINE BROMIDE tablet
- NDC Code(s): 81005-127-01, 81005-127-10, 81005-128-06, 81005-128-10
- Packager: Aarkish Pharmaceuticals NJ Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Methscopolamine Bromide Tablets, USP 2.5 mg and 5 mg contain methscopolamine bromide USP, an anticholinergic, which occurs as white crystals, or as a white odorless crystalline powder. Methscopolamine bromide melts at about 225°C with decomposition. The drug is freely soluble in water, slightly soluble in alcohol, and insoluble in acetone and in chloroform.
The chemical name for methscopolamine bromide is 3-Oxa-9-azoniatricyclo [3.3.1.0 2,4]nonane, 7-(3-hydroxy-1-oxo-2-phenylpropoxy)-9, 9-dimethyl-, bromide, [7(s)-(1α, 2β, 4β, 5α, 7β)]- and the molecular weight is 398.30.
The structural formula is represented below: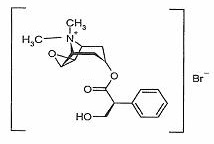
Methscopolamine Bromide Tablets, USP 2.5 mg for oral administration contain 2.5 mg of methscopolamine bromide USP. Methscopolamine Bromide Tablets, USP 5 mg for oral administration contain 5 mg of methscopolamine bromide USP.
Inactive ingredients: microcrystalline cellulose NF, pregelatinized starch NF, colloidal silicon dioxide NF, magnesium stearate NF.
Contains no lactose.
FDA approved impurity specifications differs from the USP. -
CLINICAL PHARMACOLOGY
Methscopolamine bromide is an anticholinergic agent which possesses most of the pharmacologic actions of that drug class. These include reduction in volume and total acid content of gastric secretion, inhibition of gastrointestinal motility, inhibition of salivary excretion, dilation of the pupil and inhibition of accommodation with resulting blurring of vision. Large doses may result in tachycardia.
-
PHARMACOKINETICS
Methscopolamine bromide is a quaternary ammonium derivative of scopolamine. As a class, these agents are poorly and unreliably absorbed. 1,2 Total absorption of quaternary ammonium derivatives of the alkaloids is 10 to 25%. Rate of absorption is not available. Quaternary ammonium salts have limited absorption from intact skin, and conjunctival penetration is poor. 1 Little is known of the fate and excretion of most of these agents. 1 Following oral administration, drug effects appear in about one hour and persist for 4 to 6 hours. 2 Methscopolamine bromide has limited ability to cross the blood-brain barrier. 3,4,5 The drug is excreted primarily in the urine and bile, or as unabsorbed drug in feces. 2 There is no data on the presence of methscopolamine in breast milk; traces of atropine have been found after administration of atropine. 1
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Glaucoma; obstructive uropathy (e.g., bladder neck obstruction due to prostatic hypertrophy); obstructive disease of the gastrointestinal tract (e.g., pyloroduodenal stenosis); paralytic ileus; intestinal atony of the elderly or debilitated patient; unstable cardiovascular status in acute hemorrhage; severe ulcerative colitis; toxic megacolon complicating ulcerative colitis; myasthenia gravis.
Methscopolamine Bromide Tablets, USP 2.5 mg and 5 mg is contraindicated in patients who are hypersensitive to methscopolamine bromide or related drugs. -
WARNINGS
In the presence of high environmental temperature, heat prostration (fever and heat stroke due to decreased sweating) can occur with drug use. Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance treatment with this drug would be inappropriate and possibly harmful.
Methscopolamine bromide may produce drowsiness or blurred vision. The patient should be cautioned regarding activities requiring mental alertness such as operating a motor vehicle or other machinery or performing hazardous work while taking this drug.
With overdosage, a curare-like action may occur, i.e., neuromuscular blockade leading to muscular weakness and possible paralysis. -
PRECAUTIONS
1. General precautions
Use Methscopolamine Bromide Tablets, USP 2.5 mg and 5 mg with caution in the elderly and in all patients with: autonomic neuropathy; hepatic or renal disease; or ulcerative colitis –large doses may suppress intestinal motility to the point of producing a paralytic ileus and for this reason precipitate or aggravate "toxic megacolon," a serious complication of the disease.
The drug also should be used with caution in patients having hyperthyroidism, coronary heart disease, congestive heart failure, tachyarrhythmia, tachycardia, hypertension, or prostatic hypertrophy.3. Laboratory tests
Progress of the peptic ulcer under treatment should be followed by upper gastrointestinal contrast radiology or endoscopy to insure healing. Stool tests for occult blood and blood hemoglobin or hematocrit values should be followed to rule out bleeding from the ulcer.
4. Drug interactions
Additive anticholinergic effects may result from concomitant use with antipsychotics, tricyclic antidepressants, and other drugs with anticholinergic effects. Concomitant administration with antacids may interfere with the absorption of methscopolamine bromide.
5. Carcinogenesis, mutagenesis, impairment of fertility
No long-term studies in animals have been performed to evaluate carcinogenic potential.
6. Pregnancy
Teratogenic effects
Animal reproduction studies have not been conducted with methscopolamine bromide. It is also not known whether methscopolamine bromide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Methscopolamine bromide should be given to a pregnant woman only if clearly needed. -
ADVERSE REACTIONS
The following adverse reactions have been observed, but there is not enough data to support an estimate of frequency.
Cardiovascular: Tachycardia, palpitation.
Allergic: Severe allergic reaction or drug idiosyncrasies including anaphylaxis.
CNS: Headaches, nervousness, mental confusion, drowsiness, dizziness.
Special Senses: Blurred vision, dilation of the pupil, cycloplegia, increased ocular tension, loss of taste.Renal: Urinary hesitancy and retention.
Gastrointestinal: Nausea, vomiting, constipation, bloated feeling.
Dermatologic: Decreased sweating, urticaria and other dermal manifestations.
Miscellaneous: Xerostomia, weakness, insomnia, impotence, suppression of lactation. - DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
The symptoms of overdosage with Methscopolamine Bromide Tablets, USP 2.5 mg and 5 mg progress from intensification of the usual side effects to CNS disturbances (from restlessness and excitement to psychotic behavior), circulatory changes (flushing, fall in blood pressure, circulatory failure), respiratory failure, paralysis, and coma.
Measures to be taken are (1) induction of emesis and (2) injection of physostigmine 0.5 to 2 mg intravenously, and repeated as necessary up to a total of 5 mg. Fever may be treated symptomatically (alcohol sponging, ice packs). Excitement of a degree which demands attention may be managed with sodium thiopental 2% solution given slowly intravenously or chloral hydrate (100 to 200 mL of a 2% solution) by rectal infusion. In the event of progression of the curare-like effect to paralysis of the respiratory muscles, artificial respiration should be instituted and maintained until effective respiratory action returns.
The oral LD 50in rats is 1,352 to 2,617 mg/kg.
No data is available on the dialyzability of methscopolamine bromide. -
DOSAGE AND ADMINISTRATION
The average dosage of Methscopolamine Bromide Tablets, USP is 2.5 mg one-half hour before meals and 2.5 to 5 mg at bedtime. A starting dose of 12.5 mg daily will be clinically effective in most patients without the production of appreciable side effects.
If the patient is experiencing symptoms such as severe abdominal pain or cramping which demand prompt relief, the drug may be started on a daily dosage of 20 mg, administered in doses of 5 mg one- half hour before meals and at bedtime. If very unpleasant side effects develop promptly, the daily dosage should be reduced. If neither symptomatic relief nor side effects appear, the daily dosage may be increased. Some patients have tolerated 30 mg daily with no unpleasant reactions.
Patients whose dosage has been reduced to eliminate or modify side effects often continue to show adequate response both subjectively in relief of symptoms and objectively as measured by antisecretory effects.
The ultimate aim of therapy is to arrive at a dosage which provides maximal clinical effectiveness with a minimum of unpleasant side effects. Many patients report no side effects on a dosage which gives complete relief of symptoms. On the other hand, some patients have reported severe side effects without appreciable symptomatic relief. Such patients must be considered unsuited for this therapy. Usually they have been or will prove to be similarly intolerant to other anticholinergic drugs. If methscopolamine bromide is to be used in a patient who gives a history of such intolerance, it should be started at a low dosage. -
HOW SUPPLIED
Methscopolamine Bromide Tablets, USP 2.5 mg are available as white to off-white, round shaped biconvex tablets debossed "S15" on one side of the tablet and plain on the other side, in the following package size:
Bottles of 100 (NDC 81005-127-01)
Bottles of 1000 (NDC 81005-127-10)
Methscopolamine Bromide Tablets, USP 5 mg are available as white to off-white, oval shaped biconvex tablets, debossed "S16" on one side of the tablet and plain on the other side, in the following package size:
Bottles of 60 (NDC 81005-128-06)
Bottles of 1000 (NDC 81005-128-10)Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in a tight, light- resistant container.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
-
REFERENCES
1.Gilman A, Gilman AB, Goodman LA, eds.
The Pharmacological Basis of Therapeutics. 6th ed. New York: MacMillan Publishing Company.1980.
2.American Hospital Formulary Service. American Society of Hospital Pharmacists. Bethesda, Maryland.
3.Domino EF, Corasen G. Central and Peripheral Effects of Muscarinic Cholinergic Blocking Agents in Man. Anesthesiology1967;28:568-574.
4.Mogensen L, Orinius E. Arrhythmic Complications after Parasympathetic Treatment of Bradyarrhythmias in a Coronary Care Unit. Acta Med Scand1971; 190:495-498.
5.Neeld JB Jr., et al. Cardiac Rate and Rhythm Changes with Atropine and Methscopolamine. Clin Pharmacol Ther1975;17(3):290-295.
Rx Only
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1- 800-FDA-1088
Distributed By:
Aarkish Pharmaceuticals NJ Inc.
Fairfield, NJ 07004
MADE IN USA
I-107 Rev. 00, 02/24 - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METHSCOPOLAMINE BROMIDE
methscopolamine bromide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:81005-127 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHSCOPOLAMINE BROMIDE (UNII: RTN51LK7WL) (METHSCOPOLAMINE - UNII:VDR09VTQ8U) METHSCOPOLAMINE BROMIDE 2.5 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (white to off-white) Score no score Shape ROUND Size 7mm Flavor Imprint Code S15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81005-127-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/26/2024 2 NDC:81005-127-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 02/26/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216786 02/26/2024 METHSCOPOLAMINE BROMIDE
methscopolamine bromide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:81005-128 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHSCOPOLAMINE BROMIDE (UNII: RTN51LK7WL) (METHSCOPOLAMINE - UNII:VDR09VTQ8U) METHSCOPOLAMINE BROMIDE 5 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (white to off-white) Score no score Shape ROUND Size 12mm Flavor Imprint Code S15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81005-128-06 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/26/2024 2 NDC:81005-128-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 02/26/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216786 02/26/2024 Labeler - Aarkish Pharmaceuticals NJ Inc. (117729250) Establishment Name Address ID/FEI Business Operations AACE Pharmaceuticals, Inc. 080630748 manufacture(81005-127, 81005-128)








