Label: PERPHENAZINE tablet, film coated
- NDC Code(s): 0591-4101-01, 0591-4102-01, 0591-4103-01, 0591-4104-01
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING:
Increased Mortality in Elderly Patients with Dementia-Related PsychosisElderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Perphenazine is not approved for the treatment of patients with dementia-related psychosis (see WARNINGS).
Close -
DESCRIPTION
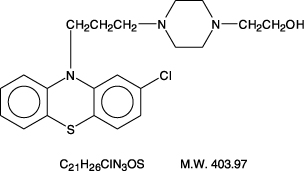
Perphenazine (4-[3-(2-chlorophenothiazin-10-yl)propyl]-1-piperazineethanol), a piperazinyl phenothiazine, having the chemical formula, C21H26CIN3OS. It is available as oral tablets containing 2 ...
-
ACTIONS
Perphenazine has actions at all levels of the central nervous system, particularly the hypothalamus. However, the site and mechanism of action of therapeutic effect are not known.
-
CLINICAL PHARMACOLOGY
Pharmacokinetics - Following oral administration of perphenazine tablets, mean peak plasma perphenazine concentrations were observed between 1 to 3 hours. The plasma elimination half-life of ...
-
INDICATIONS AND USAGE
Perphenazine is indicated for use in the treatment of schizophrenia and for the control of severe nausea and vomiting in adults. Perphenazine has not been shown effective for the management of ...
-
CONTRAINDICATIONS
Perphenazine products are contraindicated in comatose or greatly obtunded patients and in patients receiving large doses of central nervous system depressants (barbiturates, alcohol, narcotics ...
-
WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related Psychosis - Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death ...
-
PRECAUTIONS
Leukopenia, Neutropenia and Agranulocytosis - In clinical trial and postmarketing experience, events of leukopenia/ neutropenia and agranulocytosis have been reported temporally related to ...
-
ADVERSE REACTIONS
Not all of the following adverse reactions have been reported with this specific drug; however, pharmacological similarities among various phenothiazine derivatives require that each be ...
-
DOSAGE AND ADMINISTRATION
Dosage must be individualized and adjusted according to the severity of the condition and the response obtained. As with all potent drugs, the best dose is the lowest dose that will produce the ...
-
OVERDOSAGE
In the event of overdosage, emergency treatment should be started immediately. Consultation with a poison center should be considered. All patients suspected of having taken an overdose should be ...
-
HOW SUPPLIEDPerphenazine tablets USP, 2 mg are white to off white, round biconvex, film-coated tablets, debossed with “A” on one side and “280” on the other side, supplied as: NDC 0591-4101-01 bottles of 100 ...
-
SPL UNCLASSIFIED SECTIONManufactured In India By: Watson Pharma Private Limited - Verna, Salcette Goa 403 722 INDIA - Manufactured For: Teva Pharmaceuticals - Parsippany, NJ 07054 - Rev. B 3/2025
-
Principal Display PanelNDC 0591-4101-01 - Perphenazine - Tablets, USP - 2 mg - Rx only - 100 Tablets - teva
-
Principal Display PanelNDC 0591-4102-01 - Perphenazine - Tablets, USP - 4 mg - Rx only - 100 Tablets - teva
-
Principal Display PanelNDC 0591-4103-01 - Perphenazine - Tablets, USP - 8 mg - Rx only - 100 Tablets - teva
-
Principal Display PanelNDC 0591-4104-01 - Perphenazine - Tablets, USP - 16 mg - Rx only - 100 Tablets - teva
-
INGREDIENTS AND APPEARANCEProduct Information