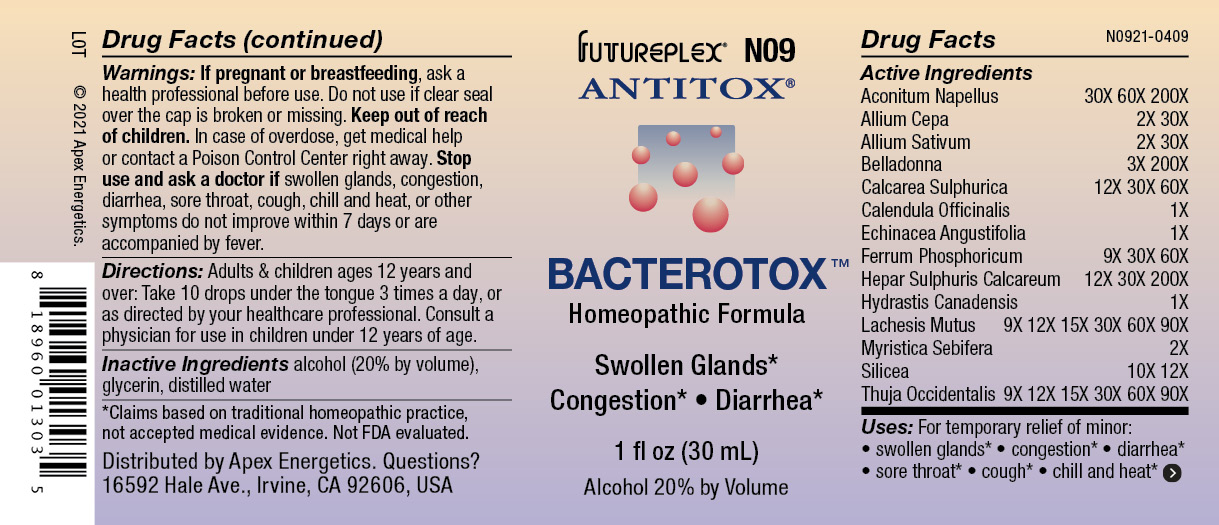
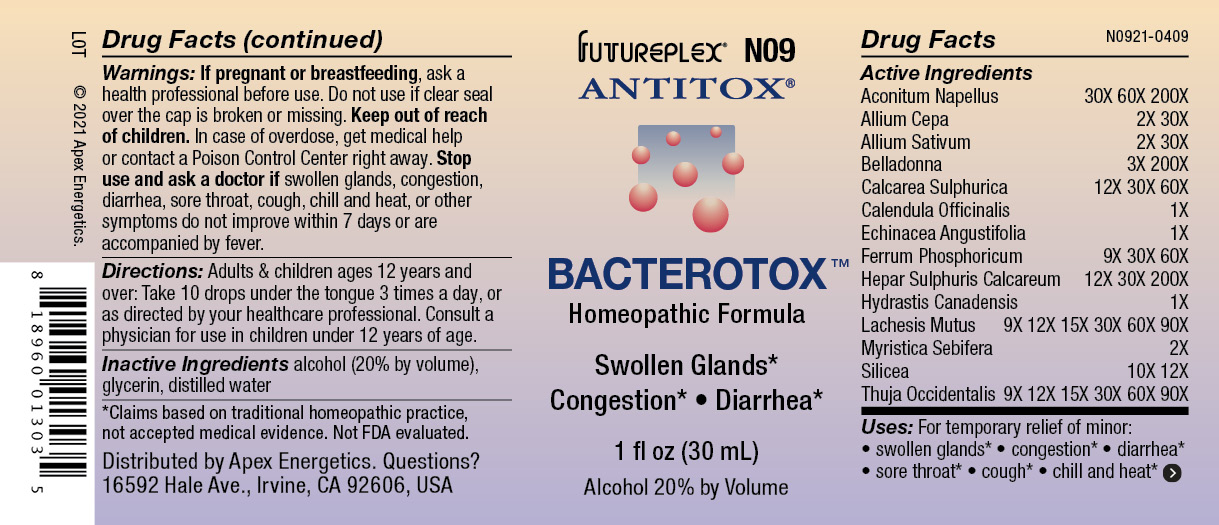
Label: N09 BACTEROTOX- aconitum napellus, allium cepa, allium sativum, belladonna, calcarea sulphurica, calendula officinalis, echinacea angustifolia, ferrum phosphoricum, calcium sulfide, hydrastis canadensis, lachesis mutus, myristica sebifera, silicea, thuja occidentalis solution/ drops
- NDC Code(s): 63479-1409-1
- Packager: Apex Energetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients
Aconitum Napellus
30X 60X 200X
Allium Cepa
2X 30X
Allium Sativum
2X 30X
Belladonna
3X 200X
Calcarea Sulphurica
12X 30X 60X
Calendula Officinalis
1X
Echinacea Angustifolia
1X
Ferrum Phosphoricum
9X 30X 60X
Hepar Sulphuris Calcareum
12X 30X 200X
Hydrastis Canadensis
1X
Lachesis Mutus
9X 12X 15X 30X 60X 90X
Myristica Sebifera
2X
Silicea
10X 12X
Thuja Occidentalis
9X 12X 15X 30X 60X 90X
- INDICATIONS & USAGE
- Warnings:
- Directions:
- Inactive Ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
N09 BACTEROTOX
aconitum napellus, allium cepa, allium sativum, belladonna, calcarea sulphurica, calendula officinalis, echinacea angustifolia, ferrum phosphoricum, calcium sulfide, hydrastis canadensis, lachesis mutus, myristica sebifera, silicea, thuja occidentalis solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63479-1409 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] in 1 mL ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 30 [hp_X] in 1 mL CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 1 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 1 [hp_X] in 1 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 200 [hp_X] in 1 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 90 [hp_X] in 1 mL FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 60 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 1 [hp_X] in 1 mL ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 200 [hp_X] in 1 mL GARLIC (UNII: V1V998DC17) (GARLIC - UNII:V1V998DC17) GARLIC 30 [hp_X] in 1 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 200 [hp_X] in 1 mL VIROLA SEBIFERA RESIN (UNII: GHJ5XX5SGS) (VIROLA SEBIFERA RESIN - UNII:GHJ5XX5SGS) VIROLA SEBIFERA RESIN 2 [hp_X] in 1 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 90 [hp_X] in 1 mL CALCIUM SULFATE ANHYDROUS (UNII: E934B3V59H) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SULFATE ANHYDROUS 60 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63479-1409-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 12/15/1994 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/15/1994 Labeler - Apex Energetics Inc. (195816384)