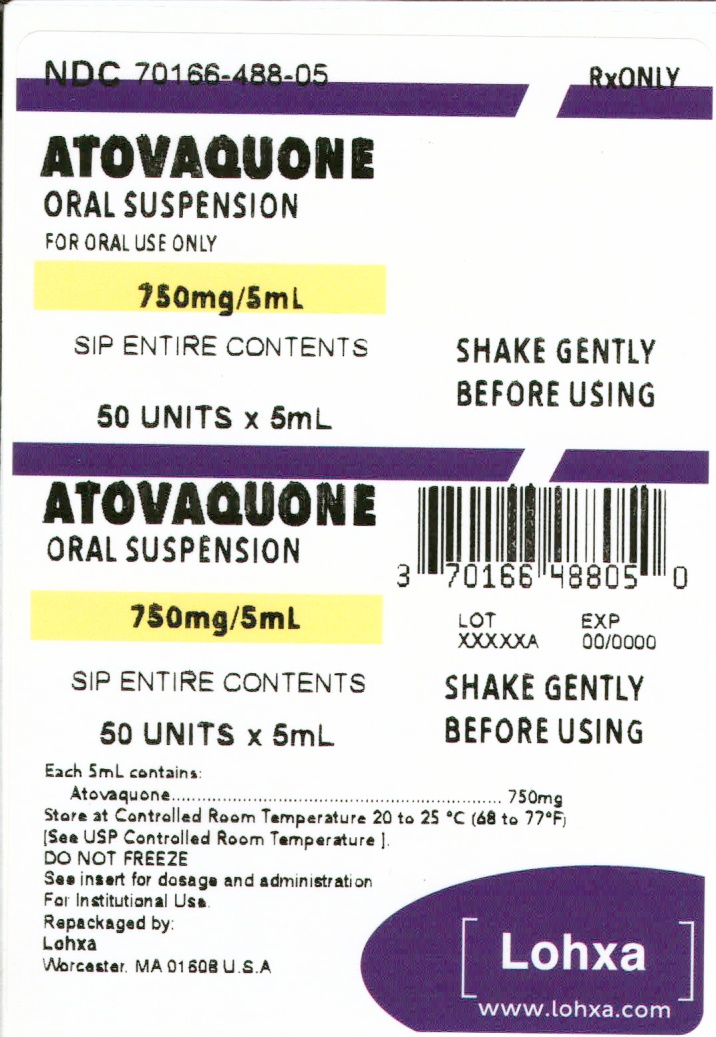
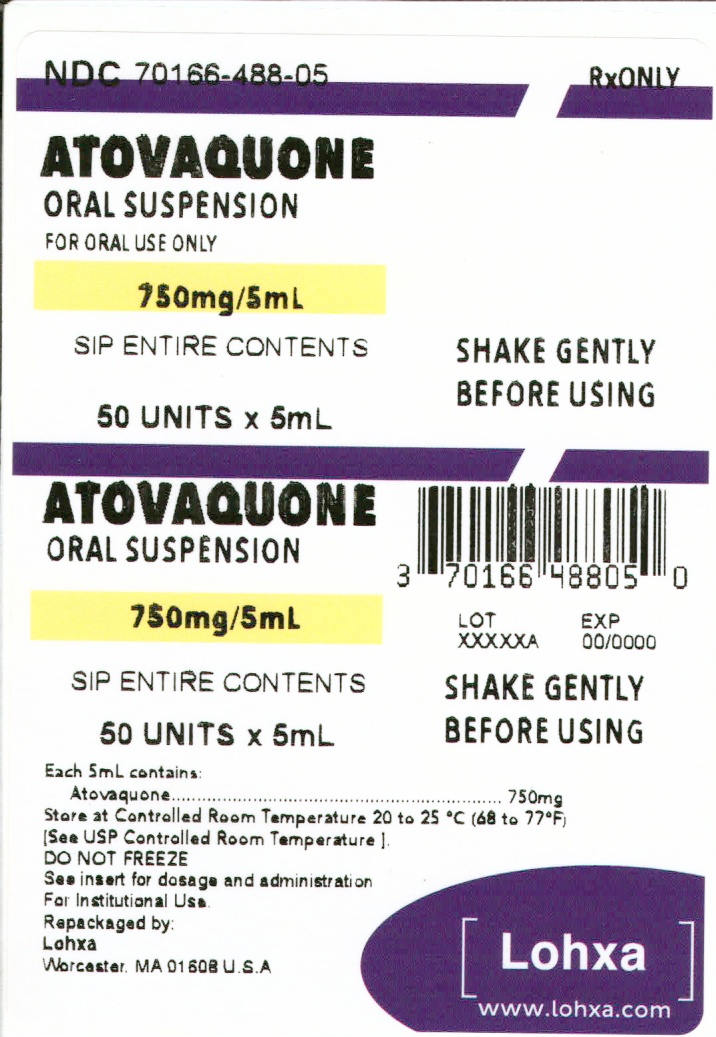
Label: ATOVAQUONE suspension
- NDC Code(s): 70166-488-05, 70166-488-10
- Packager: Lohxa
- This is a repackaged label.
- Source NDC Code(s): 31722-629
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 20, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ATOVAQUONE ORAL SUSPENSION safely and effectively. See full prescribing information for ATOVAQUONE ORAL SUSPENSION. ATOVAQUONE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS & USAGE1.1 Prevention of Pneumocystis jiroveci Pneumonia - Atovaquone oral suspension is indicated for the prevention of - Pneumocystis jirovecii pneumonia (PCP) in adults and adolescents ...
-
2 DOSAGE & ADMINISTRATION2.1 Dosage for the Prevention of P. jiroveci Pneumonia - The recommended oral dosage is 1,500 mg (10 mL) once daily administered with food. 2.2 Dosage for the Treatment of Mild-to-Moderate P ...
-
3 DOSAGE FORMS & STRENGTHSAtovaquone oral suspension, USP is a yellow homogenous suspension containing 750 mg of atovaquone USP per 5 mL.
-
4 CONTRAINDICATIONSAtovaquone oral suspension is contraindicated in patients who develop or have a history of hypersensitivity reactions (e.g., angioedema, bronchospasm, throat tightness, urticaria) to atovaquone ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Limited Oral Absorption - Absorption of orally administered atovaquone oral suspension is limited but can be significantly increased when the drug is taken with food. Failure to ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in other sections of the labeling: • Hepatotoxicity - [see Warnings and Precautions (5.2)]. 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONS7.1 Rifampin/Rifabutin - Concomitant administration of rifampin or rifabutin and atovaquone oral suspension is known to reduce atovaquone concentrations - [see Clinical ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C - There are no adequate and well-controlled studies in pregnant women. Atovaquone should be used during pregnancy only if the potential ...
-
10 OVERDOSAGEIn one patient who took an unspecified dose of dapsone, methemoglobinemia occurred. Rash has also been reported after overdose. There is no known antidote for atovaquone, and it is currently ...
-
14 CLINICAL STUDIES14.1 Prevention of PCP - The indication for prevention of PCP is based on the results of 2 clinical trials comparing atovaquone oral suspension with dapsone or aerosolized pentamidine ...
-
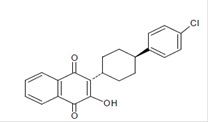
11 DESCRIPTIONAtovaquone oral suspension is a quinone antimicrobial drug. The chemical name of atovaquone is 1,4-Naphthalenedione, 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-, trans. Atovaquone USP is a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Atovaquone is a quinone antimicrobial drug - [see Clinical Pharmacology ( 12.4)]. 12.3 Pharmacokinetics - Absorption - Atovaquone is ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility - Carcinogenicity studies in rats were negative; 24-month studies in mice (dosed with 50, 100, or 200 mg/kg/day), showed ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAtovaquone oral suspension, USP is a yellow homogenous suspension containing 750 mg atovaquone USP per 5 mL. • 5mL UNIT DOSE CUPS, 50 UNITS IN 1 CARTON(NDC 70166-488-05). 5mL UNIT ...
-
17 PATIENT COUNSELING INFORMATIONAdministration Instructions - Instruct patients to: • Ensure the prescribed dose of atovaquone oral suspension is taken as directed. • Take their daily doses of ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELAtovaquone Oral Suspension, USP 750 mg/5 mL Carton Label
-
INGREDIENTS AND APPEARANCEProduct Information