Label: LIDOCAINE ointment
- NDC Code(s): 51407-383-35
- Packager: Golden State Medical Supply, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDO NOT USE IN THE EYES. Rx only
-
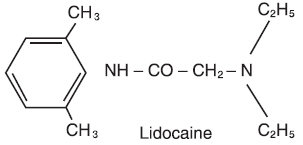
DESCRIPTIONLidocaine Ointment USP, 5% contains a local anesthetic agent and is administered topically. See - INDICATIONS AND USAGEfor specific uses. Lidocaine Ointment USP, 5% contains lidocaine, which ...
-
CLINICAL PHARMACOLOGYMechanism of action - Lidocaine stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic ...
-
INDICATIONS AND USAGELidocaine Ointment USP, 5% is indicated for production of anesthesia of accessible mucous membranes of the oropharynx. It is also useful as an anesthetic lubricant for intubation and for the ...
-
CONTRAINDICATIONSLidocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type or to other components of Lidocaine Ointment USP, 5%.
-
WARNINGSEXCESSIVE DOSAGE, OR SHORT INTERVALS BETWEEN DOSES, CAN RESULT IN HIGH PLASMA LEVELS AND SERIOUS ADVERSE EFFECTS. PATIENTS SHOULD BE INSTRUCTED TO STRICTLY ADHERE TO THE RECOMMENDED DOSAGE AND ...
-
PRECAUTIONSGeneral - The safety and effectiveness of lidocaine depend on proper dosage, correct technique, adequate precautions and readiness for emergencies. (See - WARNINGSand - ADVERSE REACTIONS. ...
-
ADVERSE REACTIONSAdverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in general ...
-
OVERDOSAGEAcute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics. (See - ADVERSE REACTIONS, WARNINGS, and ...
-
DOSAGE AND ADMINISTRATIONWhen Lidocaine Ointment USP, 5% is used concomitantly with other products containing lidocaine, the total dose contributed by all formulations must be kept in mind. Adult - A single application ...
-
HOW SUPPLIEDLidocaine Ointment USP, 5% is available in: 35.44 g (1¼ oz) laminate tube with a child-resistant cap - (NDC 51407-383-35) Store at 20° to 25°C (68° to 77°F)[see USP Controlled Room ...
-
SPL UNCLASSIFIED SECTIONMfd. by: Taro Pharmaceuticals Inc. Brampton, Ontario, Canada L6T 1C1 - Marketed by GSMS Incorporated - Camarillo, CA 93012 USA - Issued: January, 2021 - 5216711 44
-
PRINCIPAL DISPLAY PANEL - 35.44 g Tube CartonNDC 51407-383-35 - NET WT 35.44 g (1¼ oz) Lidocaine Ointment USP, 5% Laminated Tube with Child-Resistant Cap - For External Use Only - DO NOT USE IN THE EYES. Rx only - Keep this and ...
-
INGREDIENTS AND APPEARANCEProduct Information