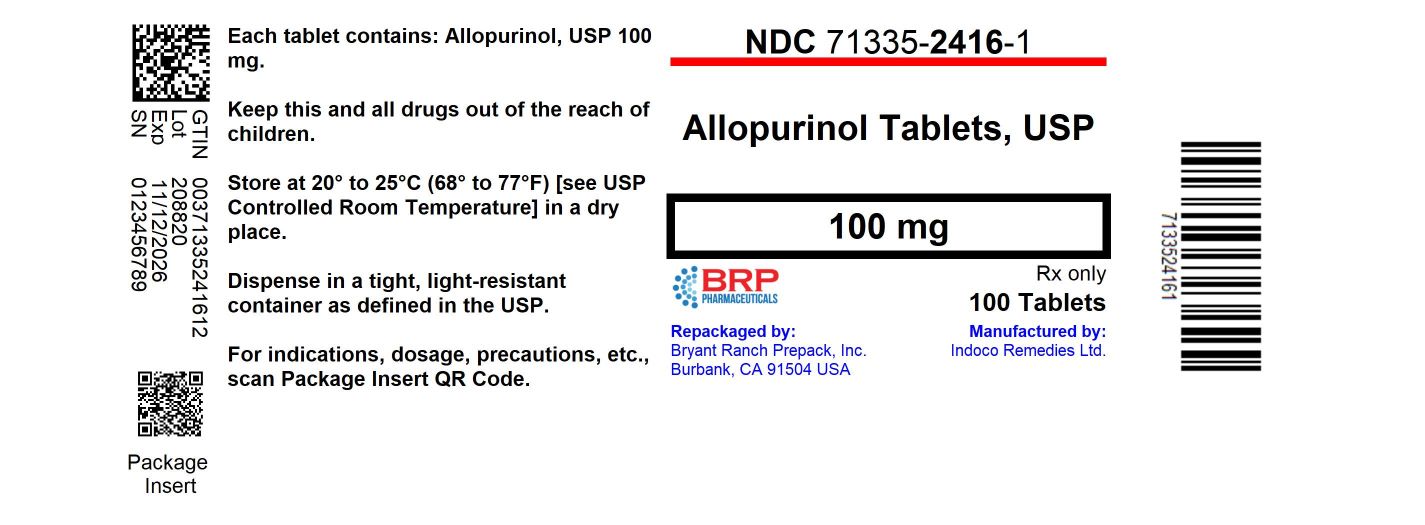
Label: ALLOPURINOL tablet
- NDC Code(s): 71335-2416-1, 71335-2416-2, 71335-2416-3, 71335-2416-4, view more
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 71921-240
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ALLOPURINOL TABLETS safely and effectively. See full prescribing information for ALLOPURINOL TABLETS. Allopurinol Tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAllopurinol Tablets are indicated for: The management of adults with signs and symptoms of primary or secondary gout (acute attacks, tophi, joint destruction, uric acid lithiasis, and/or ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Testing Prior to Treatment Initiation - Prior to initiating treatment with allopurinol tablets in patients with gout, assess the following baseline tests: serum uric acid level ...
-
3 DOSAGE FORMS AND STRENGTHSAllopurinol Tablets have functional scoring and are available in the following strengths: 100 mg: White to off white scored, flat cylindrical tablet with "I" and '135' on either side of the break ...
-
4 CONTRAINDICATIONSAllopurinol Tablets are contraindicated in patients with a history of hypersensitivity reaction to allopurinol or to any of the ingredients of allopurinol tablets.
-
5 WARNINGS AND PRECAUTIONS5.1 Skin Rash and Hypersensitivity - Serious and sometimes fatal dermatologic reactions, including toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), and drug reaction with ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: • Skin Rash and Hypersensitivity [see Warnings and Precautions (5.1)] • Nephrotoxicity [see ...
-
7 DRUG INTERACTIONS7.1 Drugs Known to Affect the Occurrence of Skin Rash and Hypersensitivity - Concomitant use of the following drugs may increase the risk of skin rash, which may be severe: bendamustine ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary Based on findings in animals, allopurinol tablets may cause fetal harm when administered to a pregnant woman. Adverse developmental outcomes have been described ...
-
10 OVERDOSAGEIn the management of overdosage there is no specific antidote for allopurinol tablets. Both allopurinol tablets and oxipurinol are dialyzable; however, the usefulness of hemodialysis or ...
-
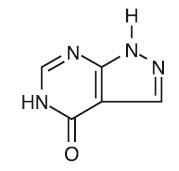
11 DESCRIPTIONAllopurinol is a xanthine oxidase inhibitor. It has the following structural formula: Allopurinol tablets are known chemically as 1, 5-dihydro-4H-pyrazolo [3, 4-d]pyrimidin-4-one and ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Allopurinol is a structural analogue of the natural purine base, hypoxanthine. Allopurinol acts on purine catabolism, without disrupting the biosynthesis of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No evidence of tumorigenicity was observed in male or female mice or rats that received oral allopurinol for the majority of their ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAllopurinol Tablets - 100 mg - White to off white scored, flat cylindrical tablet with "I" and '135' on either side of the break line on one side and plain on other side. NDC: 71335-2416-1: 100 ...
-
17 PATIENT COUNSELING INFORMATIONAdministration - Advise patients to take allopurinol tablets after meals to minimize gastric irritation. If a single dose of allopurinol tablets is occasionally forgotten, there is no need to ...
-
PRINCIPAL DISPLAY PANELAllopurinol 100mg Tablet
-
INGREDIENTS AND APPEARANCEProduct Information