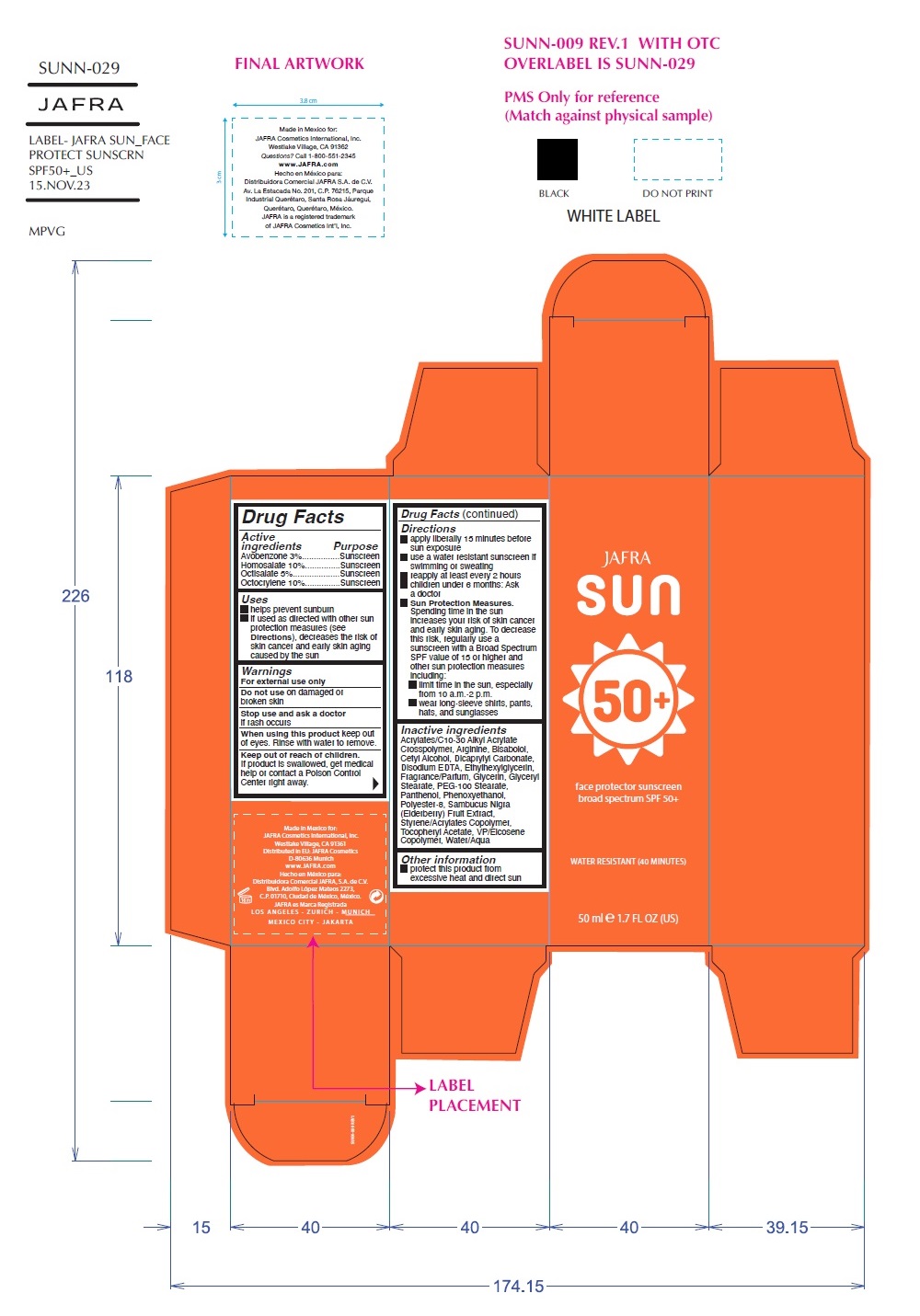
Label: SUN FACE PROTECTOR SUNSCREEN BROAD SPECTRUM SPF 50- avobenzone, homosalate, octisalate, octocrylene cream
- NDC Code(s): 68828-295-01, 68828-295-02
- Packager: Distribuidora Comercial Jafra, S.A. de C.V.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients Purpose
- Uses
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- Warnings
-
Directions
- Apply liberally and evenly 15 minutes before sun exposure
- reapply:
• after 40 minutes of swimming or sweating
• immediately after towel drying
• at least every 2 hours
- children under 6 months of age: Ask a doctor
- Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. - 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses - Other information
-
Inactive Ingredients
Water, Polyester-8, Styrene/Acrylates Copolymer, Cetyl Alcohol, VP/Eicosene Copolymer, Dicaprylyl Carbonate, Glyceryl Stearate, PEG -100 Stearate, Phenoxyethanol, Arginine, Bisabolol, Glycerin, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Tocopheryl Acetate, Fragrance, Ethylhexylglycerin, Disodium EDTA, Panthenol, Sambucus Nigra Fruit Extract, Benzyl Salicylate, Coumarin, Butylphenyl Methylpropional, Linalool, Hydroxycitronellal
- OTHER SAFETY INFORMATION
- Product label
-
INGREDIENTS AND APPEARANCE
SUN FACE PROTECTOR SUNSCREEN BROAD SPECTRUM SPF 50
avobenzone, homosalate, octisalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-295 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL Inactive Ingredients Ingredient Name Strength LEVOMENOL (UNII: 24WE03BX2T) CETYL ALCOHOL (UNII: 936JST6JCN) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PEG-100 STEARATE (UNII: YD01N1999R) PANTHENOL (UNII: WV9CM0O67Z) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) BENZYL SALICYLATE (UNII: WAO5MNK9TU) ARGININE (UNII: 94ZLA3W45F) GLYCERIN (UNII: PDC6A3C0OX) EUROPEAN ELDERBERRY (UNII: BQY1UBX046) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) WATER (UNII: 059QF0KO0R) PHENOXYETHANOL (UNII: HIE492ZZ3T) EDETATE DISODIUM (UNII: 7FLD91C86K) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) COUMARIN (UNII: A4VZ22K1WT) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) LINALOOL, (+/-)- (UNII: D81QY6I88E) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-295-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/14/2021 2 NDC:68828-295-02 7.5 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/14/2021 11/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/14/2021 Labeler - Distribuidora Comercial Jafra, S.A. de C.V. (951612777) Registrant - Jafra cosmetics international Inc. (031183599) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-295)