Label: DEXTROSE- dextrose monohydrate injection, solution
- NDC Code(s): 70518-4219-0, 70518-4219-1
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 0409-7517
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
NOTE: This solution is hypertonic - See WARNINGSand PRECAUTIONS.
LifeShield ®Abboject ®Syringe
Abboject ®Syringe
Fliptop Container
Ansyr®II Plastic Syringe
Rx only -
DESCRIPTION
50% Dextrose Injection, USP is a sterile, nonpyrogenic, hypertonic solution of dextrose in water for injection for intravenous injection as a fluid and nutrient replenisher.
Each mL of fluid contains 0.5 g dextrose, hydrous which delivers 3.4 kcal/gram. The solution has an osmolarity of 2.53 mOsmol/mL (calc.), pH 3.2 to 6.5 and may contain sodium hydroxide and/or hydrochloric acid for pH adjustment.
The solution contains no bacteriostat, antimicrobial agent or added buffer (except for pH adjustment) and is intended only for use as a single-dose injection. When smaller doses are required, the unused portion should be discarded with the entire unit.
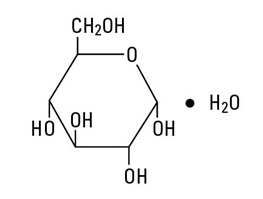
Dextrose, USP is chemically designated C 6H 12O 6∙ H 2O (D-glucose monohydrate), a hexose sugar freely soluble in water.
Dextrose, hydrous has the following structural formula:
Water for Injection, USP is chemically designated H 2O.
The syringe is molded from a specially formulated polypropylene. Water permeates from inside the container at an extremely slow rate which will have an insignificant effect on solution concentration over the expected shelf life. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the syringe material.
-
CLINICAL PHARMACOLOGY
When administered intravenously this solution restores blood glucose levels in hypoglycemia and provides a source of carbohydrate calories.
Carbohydrate in the form of dextrose may aid in minimizing liver glycogen depletion and exerts a protein-sparing action. Dextrose injection undergoes oxidation to carbon dioxide and water.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult requirement ranges from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production).
Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments and sodium (Na +) plays a major role in maintaining physiologic equilibrium.
-
INDICATIONS AND USAGE
50% Dextrose Injection is indicated in the treatment of insulin hypoglycemia (hyperinsulinemia or insulin shock) to restore blood glucose levels.
The solution is also indicated, after dilution, for intravenous infusion as a source of carbohydrate calories in patients whose oral intake is restricted or inadequate to maintain nutritional requirements. Slow infusion of hypertonic solutions is essential to ensure proper utilization of dextrose and avoid production of hyperglycemia.
-
CONTRAINDICATIONS
A concentrated dextrose solution should not be used when intracranial or intraspinal hemorrhage is present, nor in the presence of delirium tremens if the patient is already dehydrated.
Dextrose injection without electrolytes should not be administered simultaneously with blood through the same infusion set because of the possibility that pseudoagglutination of red cells may occur.
-
WARNINGS
50% Dextrose Injection is hypertonic and may cause phlebitis and thrombosis at the site of injection.
Significant hyperglycemia and possible hyperosmolar syndrome may result from too rapid administration. The physician should be aware of the symptoms of hyperosmolar syndrome, such as mental confusion and loss of consciousness, especially in patients with chronic uremia and those with known carbohydrate intolerance.
The intravenous administration of this solution can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
Additives may be incompatible. Consult with pharmacist if available. When introducing additives, use aseptic technique, mix thoroughly and do not store.
-
PRECAUTIONS
Do not use unless the solution is clear and seal is intact. Discard unused portion.
Electrolyte deficits, particularly in serum potassium and phosphate, may occur during prolonged use of concentrated dextrose solutions. Blood electrolyte monitoring is essential and fluid and electrolyte imbalances should be corrected. Essential vitamins and minerals also should be provided as needed.
To minimize hyperglycemia and consequent glycosuria, it is desirable to monitor blood and urine glucose and if necessary, add insulin.
When a concentrated dextrose infusion is abruptly withdrawn, it is advisable to follow with the administration of 5% or 10% dextrose injection to avoid rebound hypoglycemia.
Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
Care should be exercised to ensure that the needle is well within the lumen of the vein and that extravasation does not occur. If thrombosis should occur during administration, the injection should be stopped and corrective measures instituted.
Concentrated dextrose solutions should not be administered subcutaneously or intramuscularly.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Studies with solutions in polypropylene syringes have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
-
ADVERSE REACTIONS
Hyperosmolar syndrome, resulting from excessively rapid administration of concentrated dextrose may cause mental confusion and/or loss of consciousness.
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
-
OVERDOSAGE
In the event of overhydration or solute overload during therapy, re-evaluate the patient and institute appropriate corrective measures. See WARNINGSand PRECAUTIONS.
-
DOSAGE AND ADMINISTRATION
For peripheral vein administration:
Injection of the solution should be made slowly.
The maximum rate at which dextrose can be infused without producing glycosuria is 0.5 g/kg of body weight/hour. About 95% of the dextrose is retained when infused at a rate of 0.8 g/kg/hr.
In insulin-induced hypoglycemia, intravenous injection of 10 to 25 grams of dextrose (20 to 50 mL of 50% dextrose) is usually adequate. Repeated doses and supportive treatment may be required in severe cases. A specimen for blood glucose determination should be taken before injecting the dextrose. In such emergencies, dextrose should be administered promptly without awaiting pretreatment test results.
For central venous administration:
For total parenteral nutrition 50% Dextrose Injection, USP is administered by slow intravenous infusion (a) after admixture with amino acid solutions via an indwelling catheter with the tip positioned in a large central vein, preferably the superior vena cava, or (b) after dilution with sterile water for injection. Dosage should be adjusted to meet individual patient requirements.
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
The maximum rate of dextrose administration which does not result in glycosuria is the same as cited above.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. See CONTRAINDICATIONS.
-
HOW SUPPLIED
50% Dextrose Injection, USP is supplied in single-dose containers as follows:
25 g/50 mL (0.5 g/mL), Bundle of 10
50 mL Ansyr® II Plastic Syringe with syringe and barrel detached
NDC: 70518-4219-00
NDC: 70518-4219-01
PACKAGING: 10 in 1 CONTAINER
PACKAGING: 1 in 1 CARTON, 50 mL in 1 SYRINGE PLASTIC TYPE 2
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Repackaged and Distributed By:
Remedy Repack, Inc.
625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
DRUG: Dextrose
GENERIC: DEXTROSE MONOHYDRATE
DOSAGE: INJECTION, SOLUTION
ADMINSTRATION: INTRAVENOUS
NDC: 70518-4219-0
NDC: 70518-4219-1
PACKAGING: 50 mL in 1 SYRINGE, PLASTIC
OUTER PACKAGING: 1 in 1 CARTON
OUTER PACKAGING: 10 in 1 CONTAINER
ACTIVE INGREDIENT(S):
- DEXTROSE MONOHYDRATE 25g in 50mL
INACTIVE INGREDIENT(S):
- WATER
- SODIUM HYDROXIDE
- HYDROCHLORIC ACID


-
INGREDIENTS AND APPEARANCE
DEXTROSE
dextrose monohydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70518-4219(NDC:0409-7517) Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 25 g in 50 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70518-4219-0 10 in 1 CONTAINER 10/23/2024 1 1 in 1 CARTON 1 NDC:70518-4219-1 50 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019445 10/23/2024 Labeler - REMEDYREPACK INC. (829572556)