Label: DEXTROSE AND SODIUM CHLORIDE- dextrose monohydrate, sodium chloride injection, solution
- NDC Code(s): 63323-874-10
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 30, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DEXTROSE AND SODIUM CHLORIDE INJECTION safely and effectively. See full prescribing information for DEXTROSE AND SODIUM CHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
2.5% Dextrose and 0.45% Sodium Chloride Injection is indicated as a source of water, electrolytes and calories.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions - 2.5% Dextrose and 0.45% Sodium Chloride Injection is only for intravenous infusion. The osmolarity of 2.5% Dextrose and 0.45% Sodium Chloride ...
-
3 DOSAGE FORMS AND STRENGTHS
2.5% Dextrose and 0.45% Sodium Chloride Injection is a clear solution in 1000 mL single dose, flexible containers.
-
4 CONTRAINDICATIONS
2.5% Dextrose and 0.45% Sodium Chloride Injection is contraindicated in patients with: known hypersensitivity to dextrose and/or sodium chloride [see Warnings and Precautions 5.1)] clinically ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions - Hypersensitivity and infusion reactions, including anaphylaxis, have been reported with 2.5% Dextrose and 0.45% Sodium Chloride Injection [see Adverse Reactions ...
-
6 ADVERSE REACTIONS
The following adverse reactions associated with the use of 2.5% Dextrose and 0.45% Sodium Chloride Injection were identified in postmarketing reports. Because these reactions were reported ...
-
7 DRUG INTERACTIONS
7.1 Other Products that Affect Glycemic Control, Vasopressin or Fluid and/or Electrolyte Balance - 2.5% Dextrose and 0.45% Sodium Chloride Injection can affect glycemic control, vasopressin and ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Appropriate administration of 2.5% Dextrose and 0.45% Sodium Chloride Injection during pregnancy is not expected to cause adverse developmental outcomes ...
-
10 OVERDOSAGE
Excessive administration of 2.5% Dextrose and 0.45% Sodium Chloride Injection can cause: Electrolyte and Fluid Disorders - Hyperglycemia, hyperosmolality, and adverse effects on water and ...
-
11 DESCRIPTION
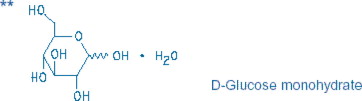
2.5% Dextrose and 0.45% Sodium Chloride Injection, USP is a sterile, nonpyrogenic solution for fluid and electrolyte replenishment and caloric supply in single-dose containers for intravenous ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - 2.5% Dextrose and 0.45% Sodium Chloride Injection is a source of water, electrolytes and calories. It is capable of inducing diuresis depending on the clinical ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
2.5% Dextrose and 0.45% Sodium Chloride is a clear solution in 1000 mL single-dose, flexible containers available as follows: Product NameSize (mL)Number per - CartonNDCProduct ...
-
17 PATIENT COUNSELING INFORMATION
Inform patients, caregivers or home healthcare providers of the following risks of 2.5% Dextrose and 0.45% Sodium Chloride Injection: Hypersensitivity reactions [see Warnings and Precautions ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY –2.5% Dextrose and 0.45% Sodium - Chloride Bag Label - NDC 63323-874-10 - freeflex® 1000 mL - 2.5% Dextrose and 0.45% Sodium Chloride Injection, USP - For ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – 2.5% Dextrose and 0.45% Sodium Chloride Case Label - NDC 63323-874-10 874010 - 2.5% Dextrose and 0.45% Sodium Chloride Injection, USP - 1000mL Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information