Label: ELIGARD- leuprolide acetate injection, suspension, extended release
- NDC Code(s): 62935-227-10, 62935-306-40, 62935-461-50, 62935-756-80
- Packager: TOLMAR Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ELIGARD® safely and effectively. See full prescribing information for ELIGARD. ELIGARD (leuprolide acetate) for injectable ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
ELIGARD is indicated for the treatment of advanced prostate cancer.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage - ELIGARD is administered subcutaneously and provides continuous release of leuprolide acetate over a one-, three-, four-, or six-month treatment period (Table 1). ELIGARD ...
-
3 DOSAGE FORMS AND STRENGTHS
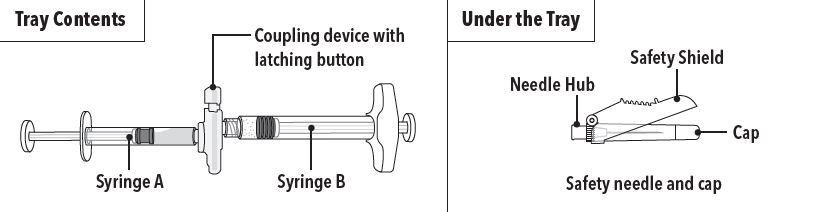
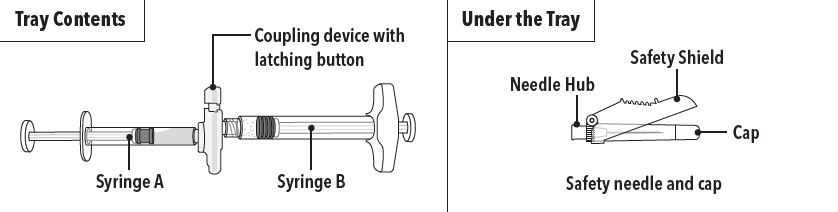
ELIGARD is an injectable suspension of leuprolide acetate available in a pre-connected syringe system and packaged with a sterile safety needle and cap (Table 2), a desiccant, prescribing ...
-
4 CONTRAINDICATIONS
Hypersensitivity - ELIGARD is contraindicated in patients with hypersensitivity to GnRH, GnRH agonist analogs or any of the components of ELIGARD. Anaphylactic reactions to synthetic GnRH or GnRH ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Tumor Flare - ELIGARD 7.5 mg, 22.5 mg, and 30 mg like other GnRH agonists, causes a transient increase in serum concentrations of testosterone during the first week of treatment. ELIGARD ...
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling: Tumor Flare [see Warnings and Precautions (5.1)] Hyperglycemia and Diabetes [see Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Based on findings in animal studies and mechanism of action, ELIGARD may cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology ...
-
11 DESCRIPTION
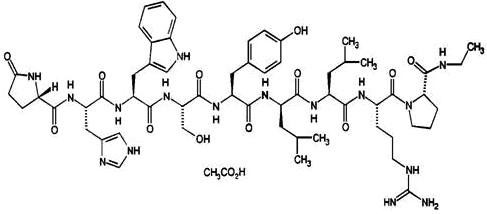
ELIGARD is a sterile polymeric matrix formulation of leuprolide acetate, a GnRH agonist, for subcutaneous injection. It is designed to deliver leuprolide acetate at a controlled rate over a one- ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Leuprolide acetate, a gonadotropin releasing hormone (GnRH) agonist, acts as an inhibitor of gonadotropin secretion when given continuously in therapeutic doses ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Two-year carcinogenicity studies were conducted with leuprolide acetate in rats and mice. In rats, a dose-related increase of ...
-
14 CLINICAL STUDIES
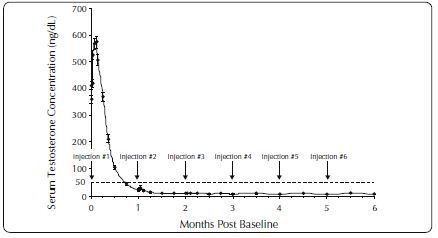
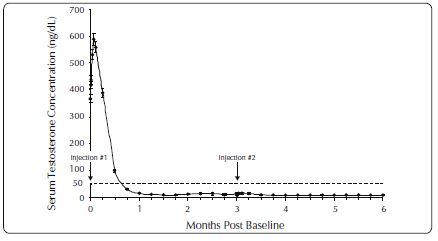
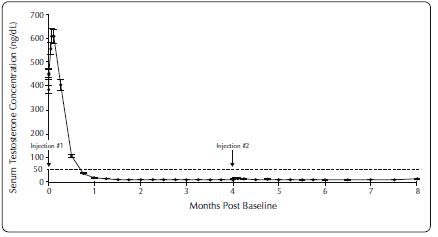
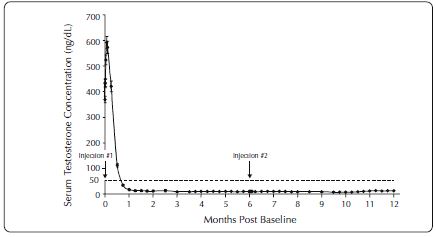
One open-label, multicenter study was conducted with each ELIGARD formulation (7.5 mg, 22.5 mg, 30 mg, and 45 mg) in patients with Jewett stage A though D prostate cancer who were treated with at ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
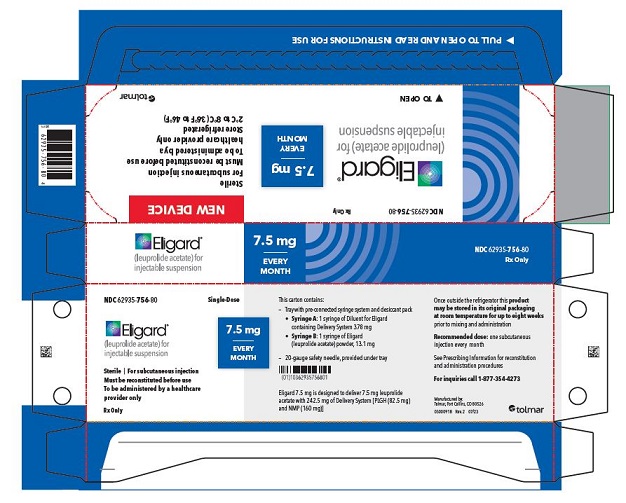
How Supplied - ELIGARD is supplied as a single-dose, two syringe-mixing system with a sterile safety needle and cap. ELIGARD is available as follows (Table 10): Table 10. ELIGARD Product ...
-
17 PATIENT COUNSELING INFORMATION
Hypersensitivity - Inform patients that if they have experienced hypersensitivity with other GnRH agonist drugs like ELIGARD, ELIGARD is contraindicated [see Contraindications (4)]. Tumor ...
-
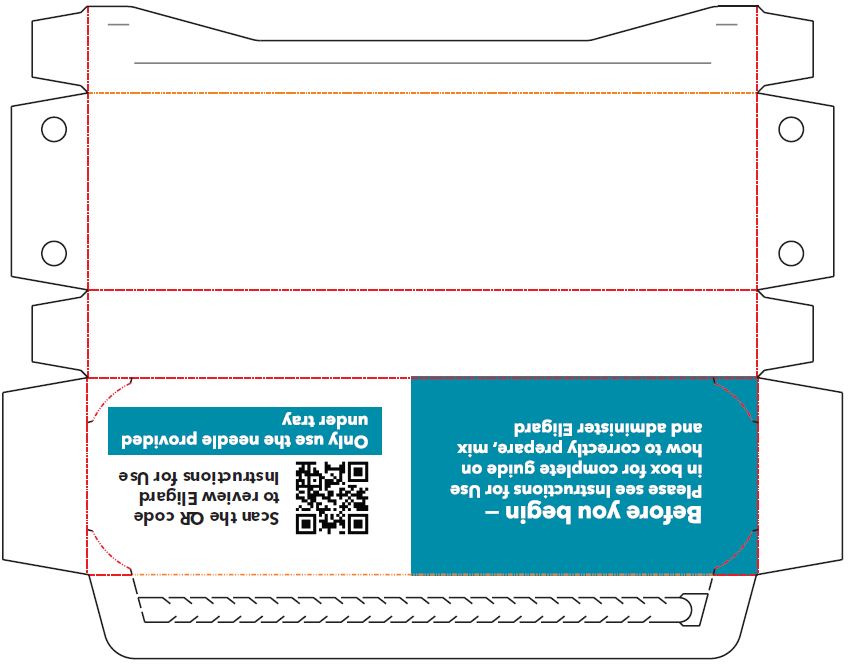
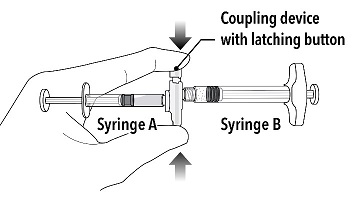
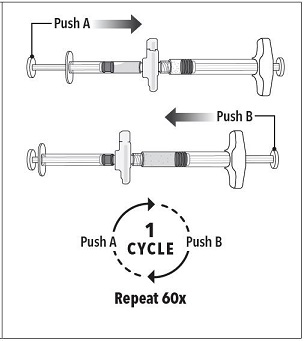
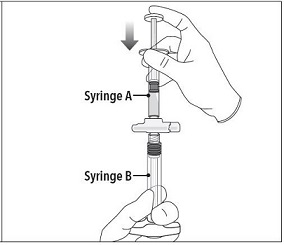
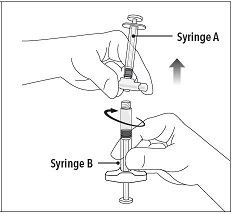
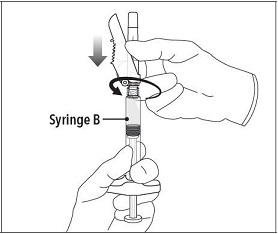
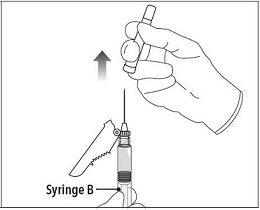
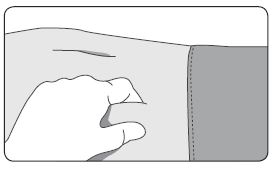
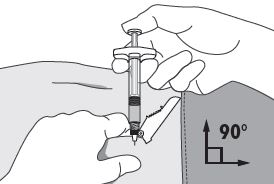
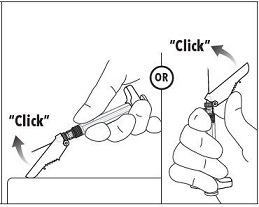
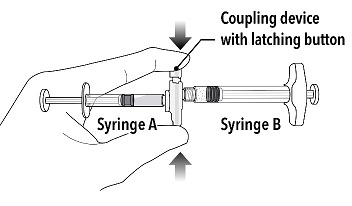
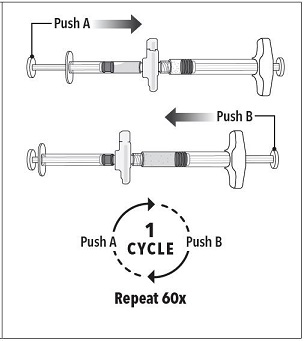
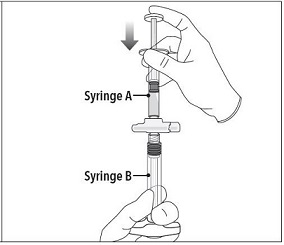
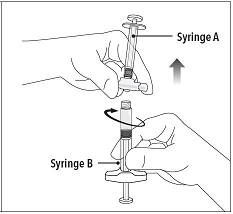
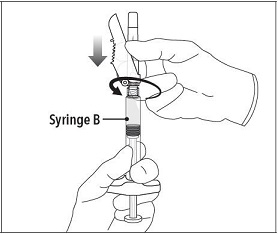
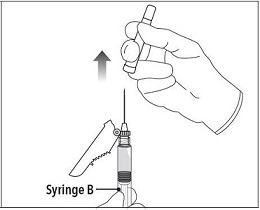
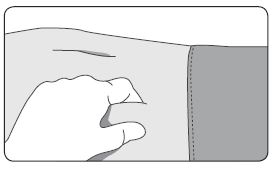
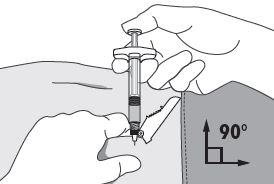
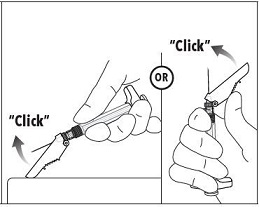
INSTRUCTIONS FOR USEFollow the detailed instructions below to ensure correct preparation of ELIGARD prior to administration: Step 1 - Use aseptic technique throughout the procedure. Gloves are recommended ...
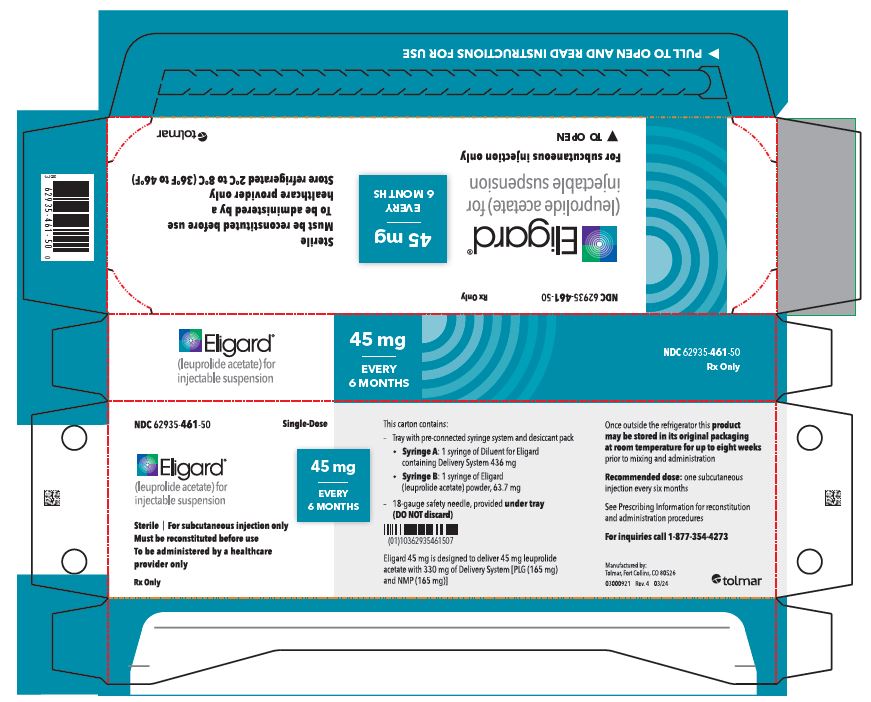
- PRINCIPAL DISPLAY PANEL
-
7.5 mg carton
 ...
... -
22.5 mg carton
 ...
... -
30 mg carton
 ...
... -
45 mg carton
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information