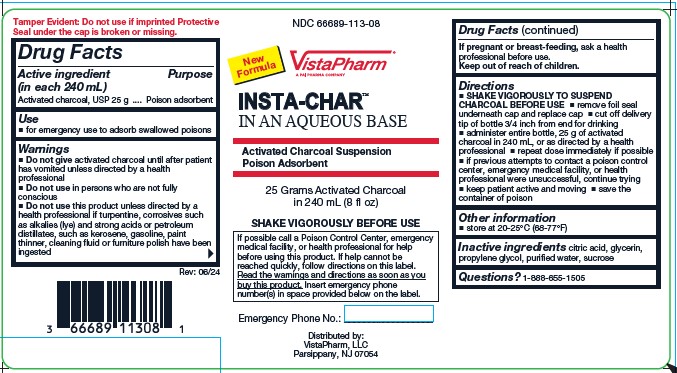
Label: INSTA-CHAR AQUEOUS- poison treatment adsorbent suspension
- NDC Code(s): 66689-113-08
- Packager: VistaPharm, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (per 240 mL)
- Purpose
- Uses
-
Warnings
Do not use
- Do not give activated charcoal until after patient has vomited unless directed by a health professional
- Do not use in persons who are not fully conscious
- Do not use this product unless directed by a health professional if turpentine, corrosives such as alkalies (lye) and strong acids or petroleum distillates, such as kerosene, gasoline, paint thinner, cleaning fluid or furniture polish have been ingested.
-
Directions
- SHAKE VIGOROUSLY TO SUSPEND CHARCOAL BEFORE USE.
- remove foil seal underneath cap and replace cap.
- cut off delivery tip of bottle 3/4 inch from end for drinking.
- administer entire bottle, 25 g of activated charcoal in 240 mL, or as directed by a health professional
- repeat dose immediately if possible.
- if previous attempts to contact a poison control center, emergency medical facility, of health professional were unsuccessful, continue trying.
- keep patient active and moving.
- save the container of poison.
- Other Information
- Inactive Ingredients
- Questions?
- Insta-Char Aqueous 240 mL
-
INGREDIENTS AND APPEARANCE
INSTA-CHAR AQUEOUS
poison treatment adsorbent suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66689-113 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 50 g in 240 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66689-113-08 240 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/13/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/13/2024 Labeler - VistaPharm, LLC (048458728)