Label: CHLORPROMAZINE HYDROCHLORIDE injection
- NDC Code(s): 73043-048-01, 73043-048-25, 73043-049-01, 73043-049-25
- Packager: Devatis, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 12, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
BOXED WARNING
(What is this?)
WARNING
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Chlorpromazine Hydrochloride Injection is not approved for the treatment of patients with dementia-related psychosis (see WARNINGS).
Close -
DESCRIPTION
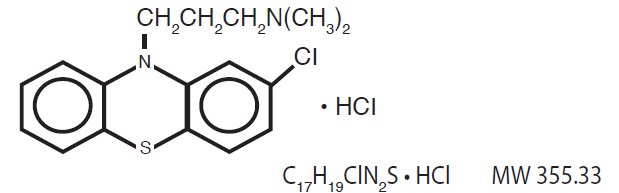
Chlorpromazine HCl is chemically designated as 2-Chloro-10-[3-(dimethylamino)propyl]-phenothiazine monohydrochloride and has the following structural formula: Chlorpromazine Hydrochloride ...
-
CLINICAL PHARMACOLOGY
The precise mechanism whereby the therapeutic effects of chlorpromazine are produced is not known. The principal pharmacological actions are psychotropic. It also exerts sedative and antiemetic ...
-
INDICATIONS AND USAGE
For the treatment of schizophrenia; to control nausea and vomiting; for relief of restlessness and apprehension before surgery; for acute intermittent porphyria; as an adjunct in the treatment ...
-
CONTRAINDICATIONS
Do not use in patients with known hypersensitivity to phenothiazines. Do not use in comatose states or in the presence of large amounts of central nervous system depressants (alcohol ...
-
WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related Psychosis - Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death ...
-
PRECAUTIONS
Leukopenia, Neutropenia and Agranulocytosis - In clinical trial and postmarketing experience, events of leukopenia/neutropenia and agranulocytosis have been reported temporally related to ...
-
ADVERSE REACTIONS
Note: Some adverse effects of chlorpromazine may be more likely to occur, or occur with greater intensity, in patients with special medical problems, e.g., patients with mitral insufficiency or ...
-
OVERDOSAGE
(See also ADVERSE REACTIONS.) Symptoms - Primarily symptoms of central nervous system depression to the point of somnolence or coma. Hypotension and extrapyramidal symptoms. Other possible ...
-
DOSAGE AND ADMINISTRATION
Adults - Adjust dosage to individual and the severity of his condition, recognizing that the milligram for milligram potency relationship among all dosage forms has not been precisely ...
-
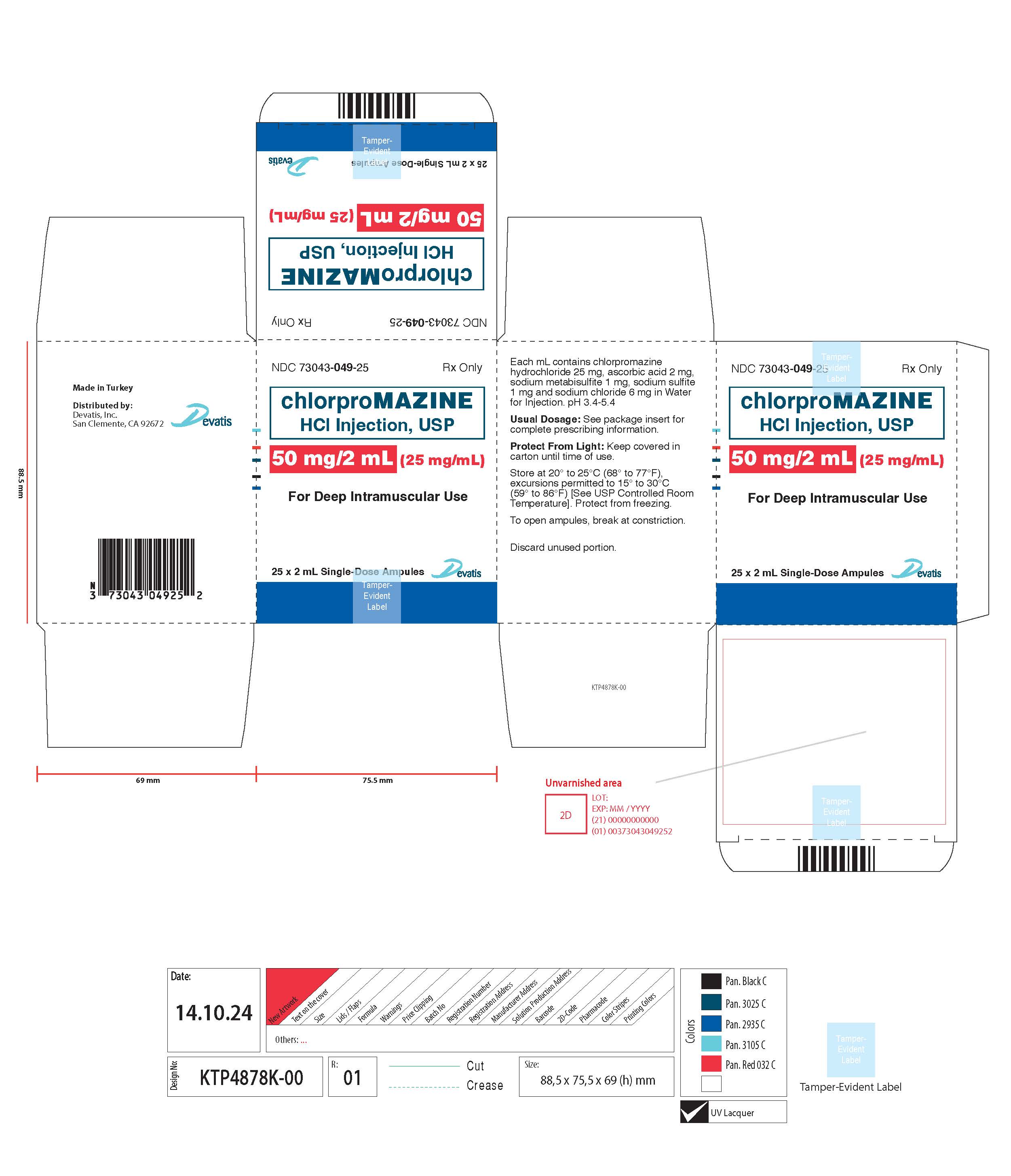
HOW SUPPLIED
Chlorpromazine Hydrochloride Injection USP, 25 mg/mL and 50 mg/2 mL (25 mg/mL), are available in the following packages: 1 mL Single-Dose Ampule packaged in 25s (NDC 73043 048 25) 2 mL Single-Dose ...
-
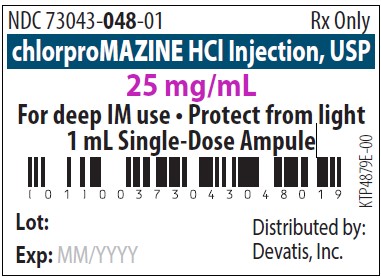
PRINCIPAL DISPLAY PANEL
NDC 73043-048-01 Rx only - chlorproMAZINE HCl Injection, USP - 25 mg/mL - For deep IM use - Protect from light - 1 mL Single-Dose Ampule - Distributed by: Devatis, Inc. NDC 73043-048-25 Rx ...
-
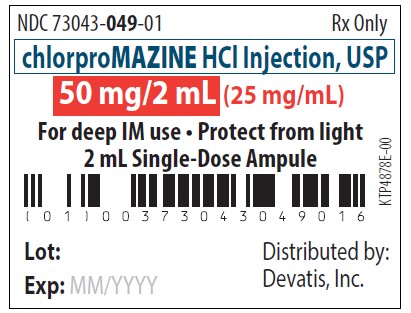
PRINCIPAL DISPLAY PANEL
NDC 73043-049-01Rx only - chlorproMAZINE HCl Injection, USP - 50 mg/2 mL (25 mg/mL) For deep IM use - Protect from light - 2 mL Single-Dose Ampule - Distributed by: Devatis, Inc. NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information