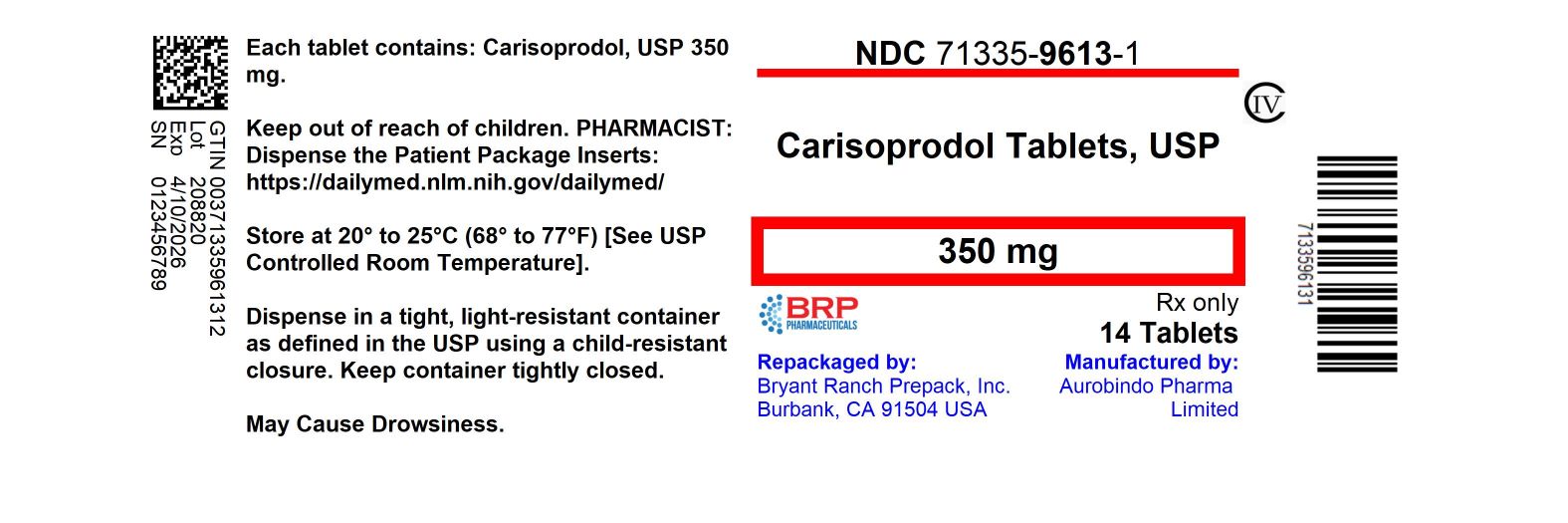
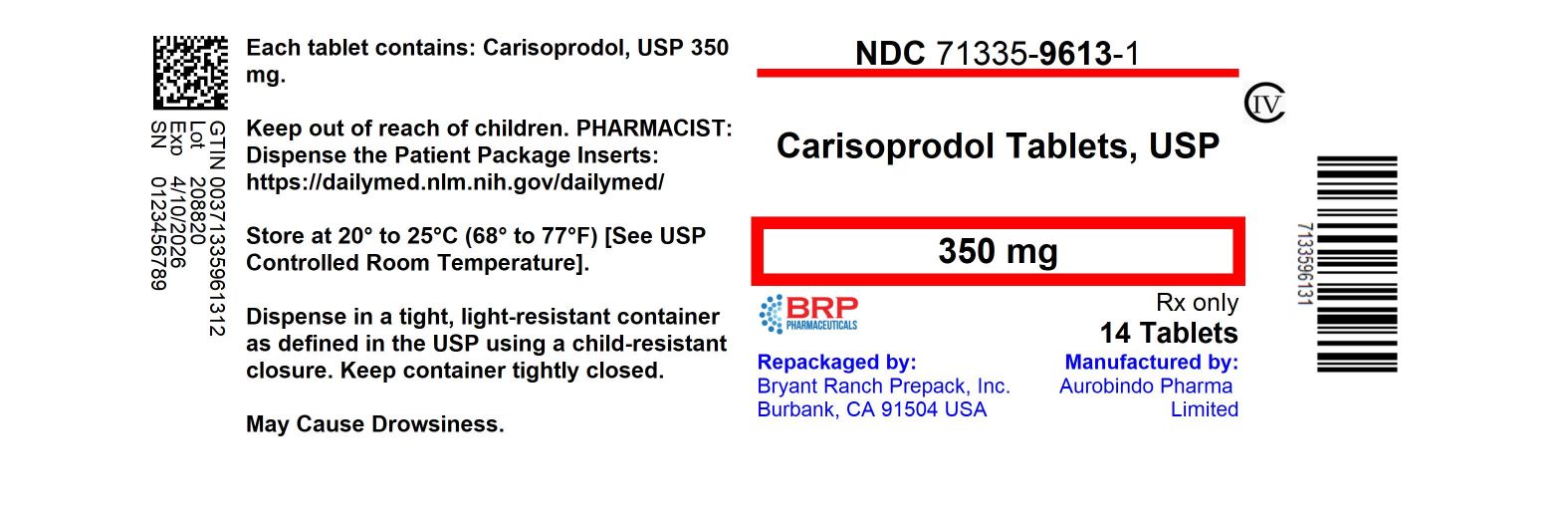
Label: CARISOPRODOL tablet
- NDC Code(s): 71335-9613-0, 71335-9613-1, 71335-9613-2, 71335-9613-3, view more
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 16571-781
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CARISOPRODOL TABLETS safely and effectively. See full prescribing information for CARISOPRODOL TABLETS. CARISOPRODOL tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECarisoprodol tablets are indicated for the relief of discomfort associated with acute, painful musculoskeletal conditions in adults. Limitation of Use - Carisoprodol tablets should only be ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dose of carisoprodol tablets is 250 mg to 350 mg three times a day and at bedtime. The recommended maximum duration of carisoprodol tablets use is up to two or three weeks.
-
3 DOSAGE FORMS AND STRENGTHSCarisoprodol tablets USP, 250 mg are white colored, round shaped, biconvex, uncoated tablets, identified with ‘H 94’ debossed on one side and other side plain. Carisoprodol tablets USP, 350 mg ...
-
4 CONTRAINDICATIONSCarisoprodol tablets are contraindicated in patients with a history of acute intermittent porphyria or a hypersensitivity reaction to a carbamate such as meprobamate.
-
5 WARNINGS AND PRECAUTIONS5.1 Sedation - Carisoprodol has sedative properties (in the low back pain trials, 13% to 17% of patients who received carisoprodol experienced sedation compared to 6% of patients who received ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in clinical studies of a drug cannot be directly compared ...
-
7 DRUG INTERACTIONS7.1 CNS Depressants - The sedative effects of carisoprodol and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) may be additive. Therefore, caution ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Data over many decades of carisoprodol use in pregnancy have not identified a drug-associated risk of major birth defects, miscarriage, or other adverse maternal ...
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - Carisoprodol, a Schedule IV controlled substance. Carisoprodol has been subject to abuse, misuse, and criminal diversion for nontherapeutic use [see Warnings and ...
-
10 OVERDOSAGEClinical Presentation - Overdosage of carisoprodol commonly produces CNS depression. Death, coma, respiratory depression, hypotension, seizures, delirium, hallucinations, dystonic reactions ...
-
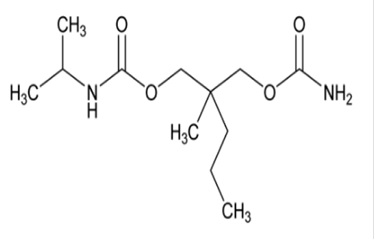
11 DESCRIPTIONCarisoprodol tablets, USP are available as 250 mg and 350 mg round, white tablets. Carisoprodol USP is a white, crystalline powder, having a mild, characteristic odor and a bitter taste. It is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of carisoprodol in relieving discomfort associated with acute painful musculoskeletal conditions has not been clearly identified. In animal ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long term studies in animals have not been performed to evaluate the carcinogenic potential of ...
-
14 CLINICAL STUDIESThe safety and efficacy of carisoprodol for the relief of acute, idiopathic mechanical low back pain was evaluated in two, 7-day, double blind, randomized, multicenter, placebo controlled, U.S ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCarisoprodol Tablets USP, 350 mg are white colored, round shaped, biconvex, uncoated tablets, identified with ‘D’ debossed on one side and ‘31’ on the other side. NDC: 71335-9613-1: 14 Tablets in ...
-
17 PATIENT COUNSELING INFORMATIONPatients should be advised to contact their physician if they experience any adverse reactions to carisoprodol. Sedation - Advise patients that carisoprodol may cause drowsiness and/or ...
-
PRINCIPAL DISPLAY PANELCarisoprodol 350mg (CIV) Tablet
-
INGREDIENTS AND APPEARANCEProduct Information