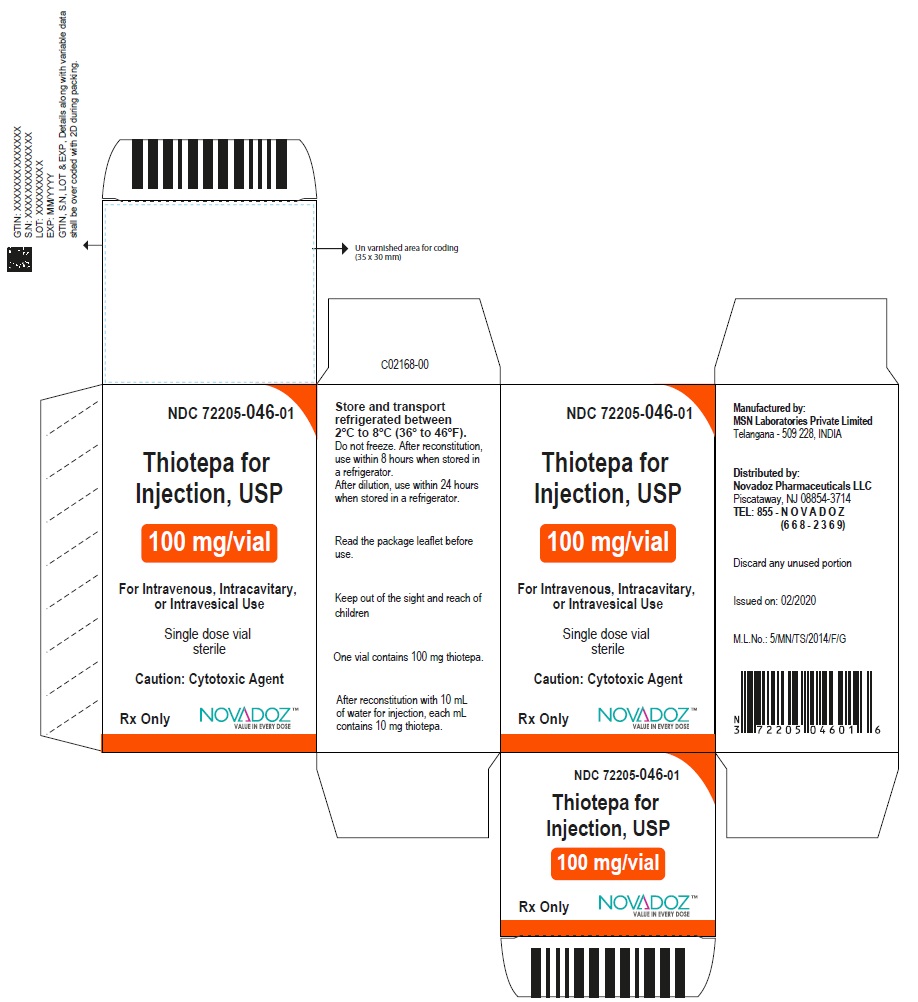
Label: THIOTEPA injection, powder, lyophilized, for solution
- NDC Code(s): 72205-045-01, 72205-046-01
- Packager: Novadoz Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use THIOTEPA FOR INJECTION safely and effectively. See full prescribing information for THIOTEPA FOR INJECTION. THIOTEPA for injection ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SEVERE MYELOSUPPRESSION, CARCINOGENICITY
Close
• Thiotepa may cause severe marrow suppression, and high doses may cause marrow ablation with resulting infection or bleeding. Monitor hematologic laboratory parameters. Hematopoietic progenitor (stem) cell transplantation (HSCT) is required to prevent potentially fatal complications of the prolonged myelosuppression after high doses of thiotepa [see Warnings and Precautions (5.1)]
• Thiotepa should be considered potentially carcinogenic in humans [see Warnings and Precautions (5.7)] -
1 INDICATIONS AND USAGE1.1 Class 3 Beta-Thalassemia - Thiotepa for injection is indicated to reduce the risk of graft rejection when used in conjunction with high-dose busulfan and cyclophosphamide as a preparative ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Class 3 Beta-Thalassemia - The recommended dose of thiotepa for injection in pediatric patients is two administrations of 5 mg/kg given intravenously approximately 12 ...
-
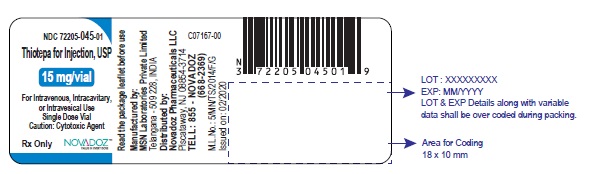
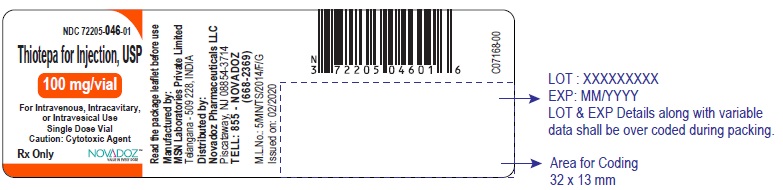
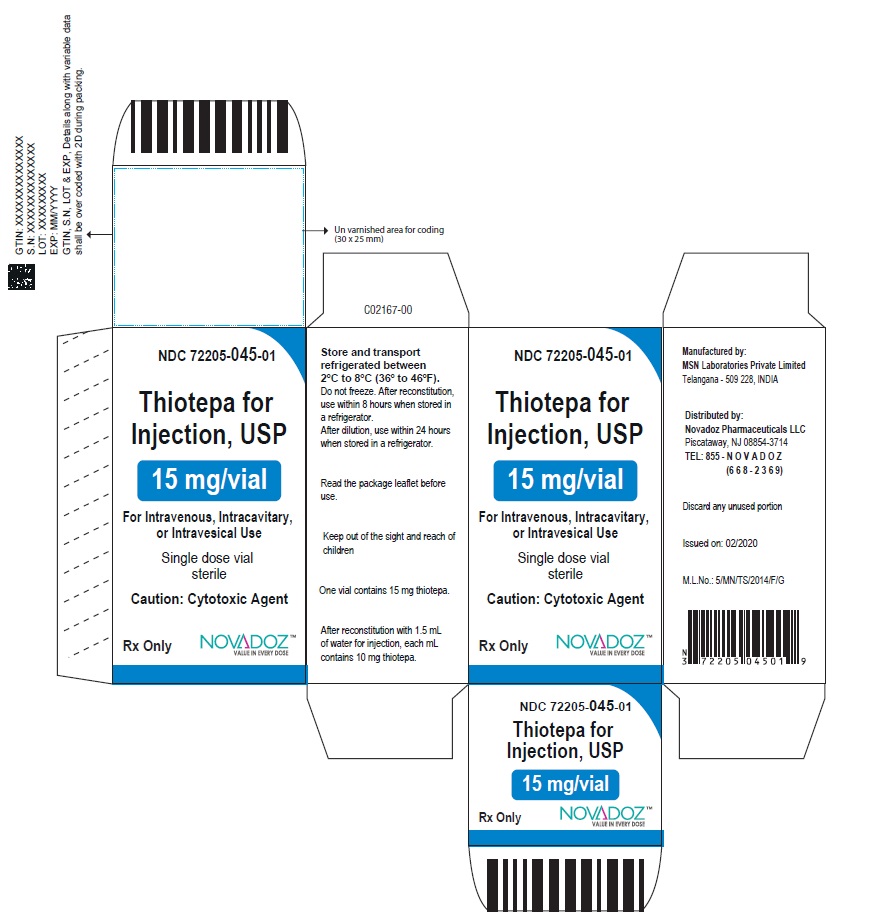
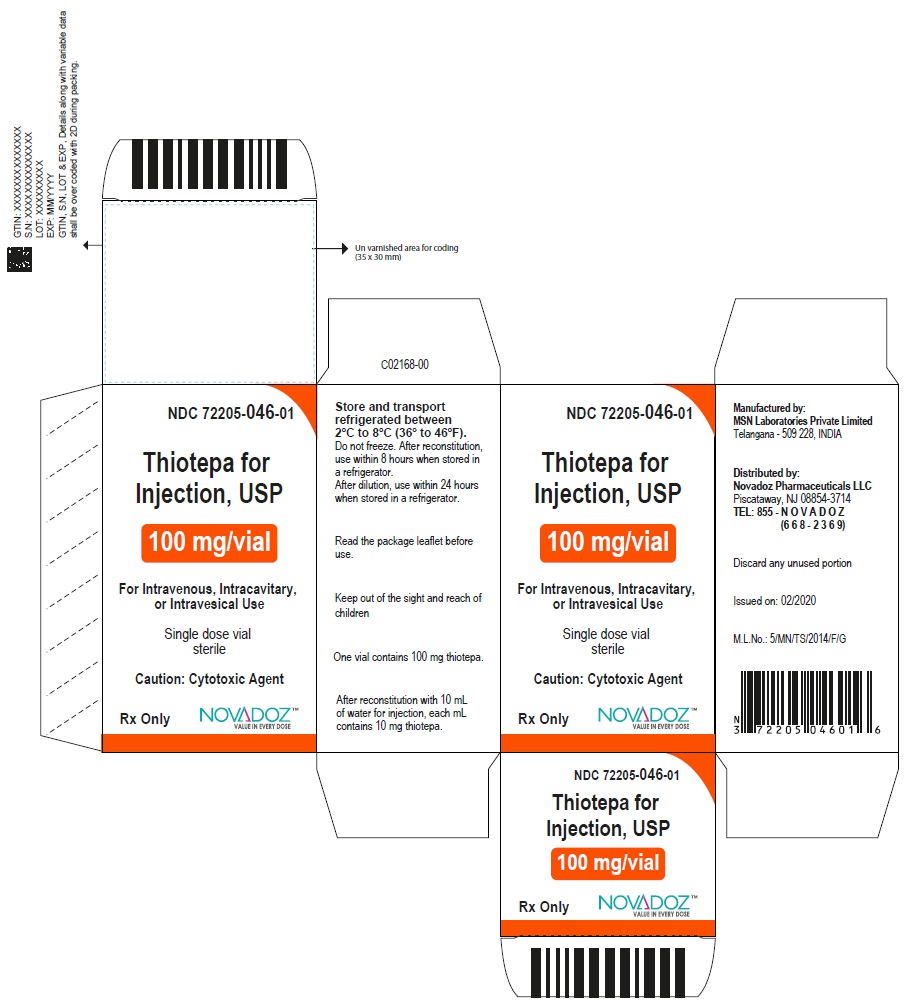
3 DOSAGE FORMS AND STRENGTHSFor injection, 15 mg, lyophilized white powder in single-dose vial for reconstitution - For injection, 100 mg, lyophilized white powder in single-dose vial for reconstitution
-
4 CONTRAINDICATIONSThiotepa for injection is contraindicated in: Patients with severe hypersensitivity to thiotepa [see Warnings and Precautions (5.2)] Concomitant use with live or attenuated vaccines [see ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression - The consequence of treatment with high doses of thiotepa together with other chemotherapy at the recommended dose and schedule in the preparative regimen for class 3 ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Myelosuppression [see Warnings and Precautions (5.1)] Infection [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Effect of Cytochrome CYP3A Inhibitors and Inducers - In vitro studies suggest that thiotepa is metabolized by CYP3A4 and CYP2B6 to its active metabolite TEPA. Avoid coadministration of strong ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Thiotepa can cause fetal harm when administered to a pregnant woman based on findings from animals and the drug’s mechanism of action [see Clinical Pharmacology ...
-
10 OVERDOSEThere is no experience with overdoses of thiotepa. The most important adverse reactions expected in case of overdose are myeloablation and pancytopenia [see Nonclinical Toxicology (13.2)]. There ...
-
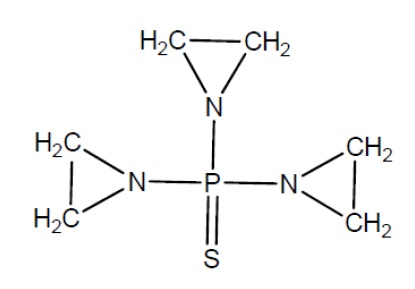
11 DESCRIPTIONThiotepa is an alkylating agent. Thiotepa for injection, USP is supplied as a non-pyrogenic, sterile lyophilized white powder for intravenous, intracavitary, or intravesical use after ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Thiotepa is a cytotoxic agent of the polyfunctional type, related chemically and pharmacologically to the nitrogen mustard. The radiomimetic action of thiotepa is ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In mice, repeated intraperitoneal (IP) administration of thiotepa (1.15 or 2.3 mg/kg three times per week for 52 or 43 weeks ...
-
14 CLINICAL STUDIESThiotepa was evaluated in a retrospective study of pediatric patients with class 3 beta-thalassemia who underwent allogeneic hematopoietic progenitor (stem) cell transplantation (HSCT) from a ...
-
15 REFERENCES1. OSHA Hazardous Drugs. OSHA. [Accessed from - http://www.osha.gov/SLTC/hazardousdrugs/index.html].
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Thiotepa for injection, USP is supplied as Unit carton with one single-dose Type I clear glass vial. The vial stopper is not made with natural rubber latex. Thiotepa for ...
-
17 PATIENT COUNSELING INFORMATIONHypersensitivity - Counsel patients on the signs and symptoms of hypersensitivity and to seek immediate emergency assistance if they develop any of these signs and symptoms [see Warnings and ...
-
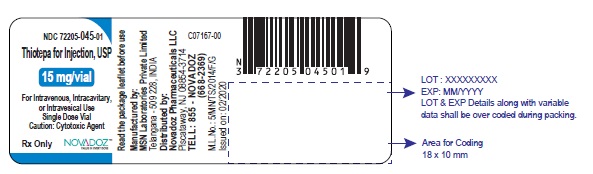
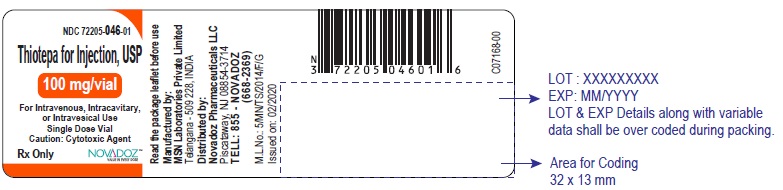
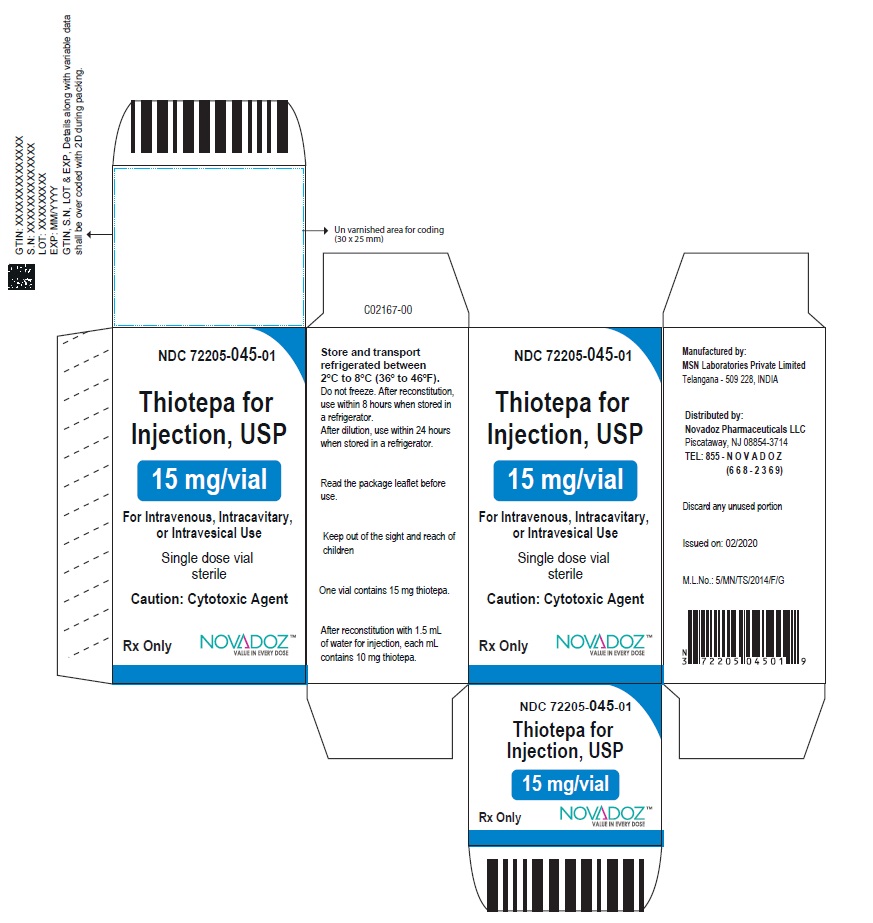
PACKAGE LABEL.PRINCIPAL DISPLAY PANELThiotepa-for-Injection-15mg-vial-label - Thiotepa-for-Injection-100mg-vial-label - Thiotepa-for-Injection-15mg-carton-label - Thiotepa-for-Injection-100mg-carton-label
-
INGREDIENTS AND APPEARANCEProduct Information