Label: RHINASE D GEL MOISTURIZING NASAL DECONGESTANT- oxymetazoline hcl 0.5% jelly
- NDC Code(s): 69051-202-01
- Packager: Profounda Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient........Purpose
- ASK DOCTOR
- PURPOSE
-
WHEN USING
- do not use more than directed
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- use of this container by more than one person may spread infection.
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- QUESTIONS
-
DOSAGE & ADMINISTRATION
Adults and Children 6 to under 12 years of age (with adult supervision) Place a small amount in each nostril and inhale well back into the nasal passages, not more often than every 10-12 hours. Do not exceed 2 doses in any 24 hour period.
children under 6 years of age ask a doctor.
To Use: press down grooved white cap firmly and turn counter clockwise . Secure cap after use.
- WARNINGS
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- INDICATIONS & USAGE
- RhinaseD_Sachet_3g_200422_052920-FIN.jpg
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RHINASE D GEL MOISTURIZING NASAL DECONGESTANT
oxymetazoline hcl 0.5% jellyProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69051-202 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 0.05 {VP} in 100 {VP} Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) 0.7 {VP} in 100 {VP} WATER (UNII: 059QF0KO0R) 55.5 {VP} in 100 {VP} BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) 0.018 {VP} in 100 {VP} POTASSIUM CHLORIDE (UNII: 660YQ98I10) 0.167 {VP} in 100 {VP} SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.218 {VP} in 100 {VP} POLYETHYLENE GLYCOL 10000 (UNII: H57W405143) 14.993 {VP} in 100 {VP} DIPROPYLENE GLYCOL (UNII: E107L85C40) 19.99 {VP} in 100 {VP} CARBOMER 934 (UNII: Z135WT9208) 1.809 {VP} in 100 {VP} SODIUM HYDROXIDE (UNII: 55X04QC32I) 0.308 {VP} in 100 {VP} Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69051-202-01 3 in 1 TUBE 12/21/2020 1 3 {VP} in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 12/21/2020 Labeler - Profounda Inc (078862060) Registrant - Profounda Inc (078862060) Establishment Name Address ID/FEI Business Operations Profounda Inc 078862060 manufacture(69051-202)


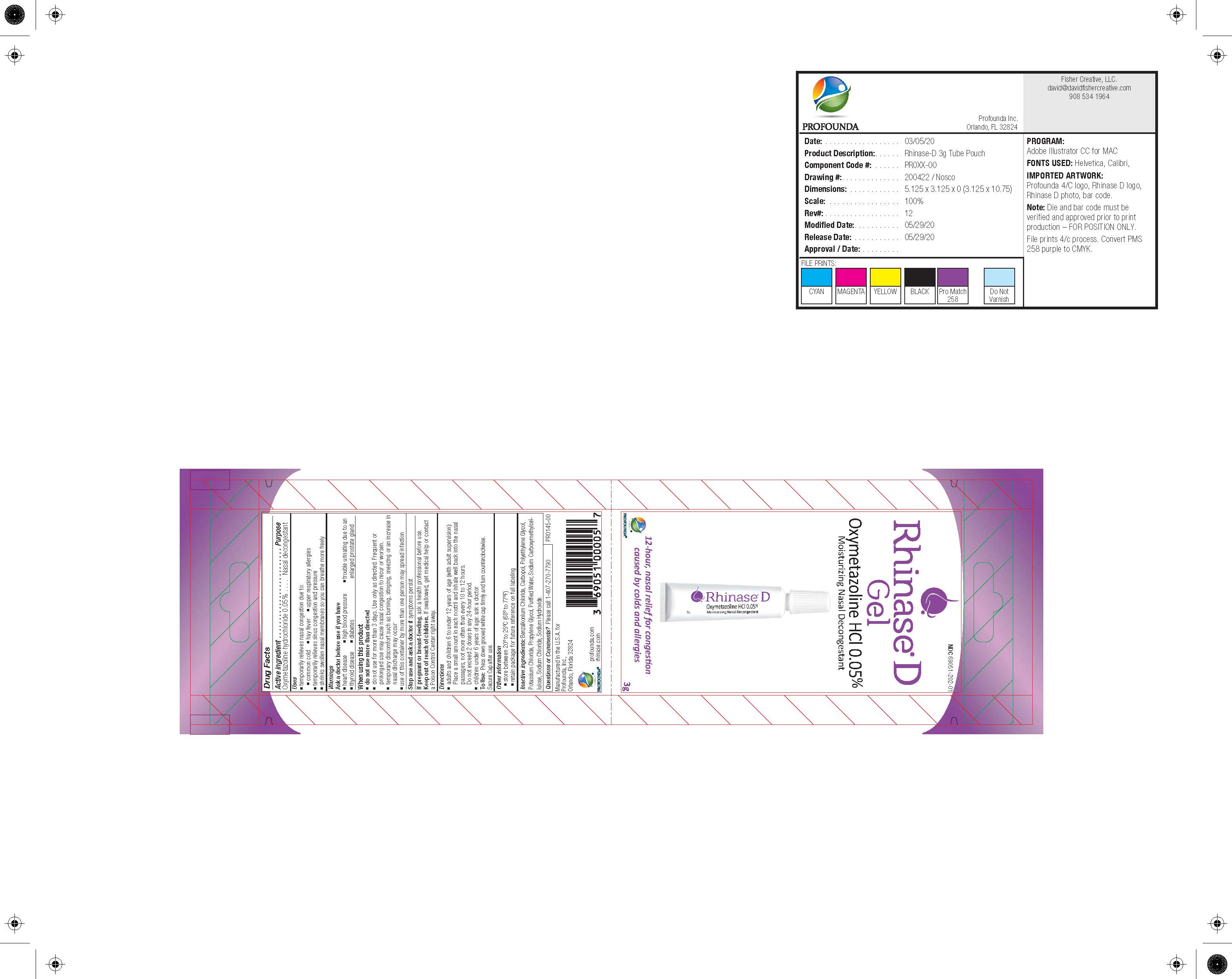 NDC 69051-202-01
NDC 69051-202-01