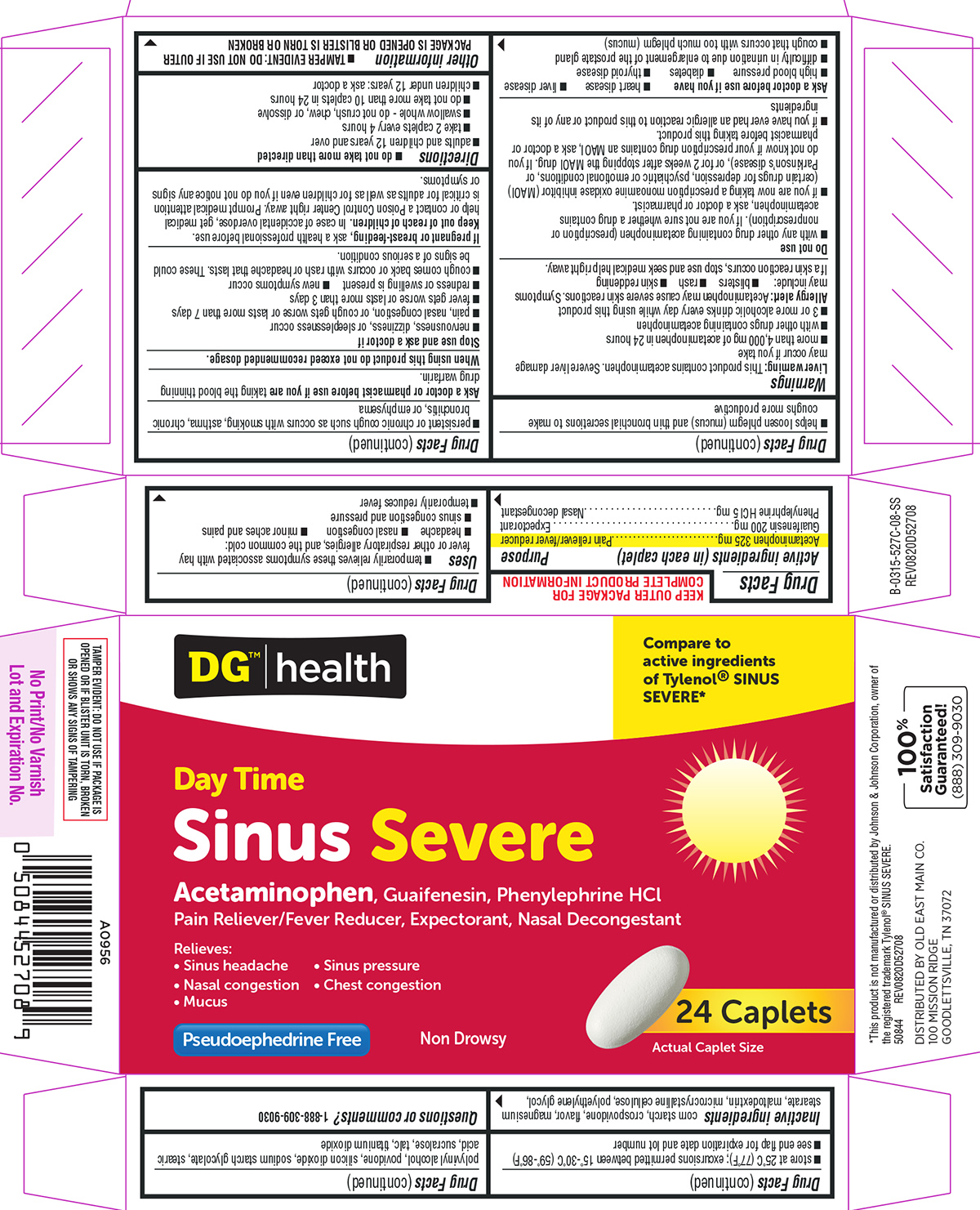
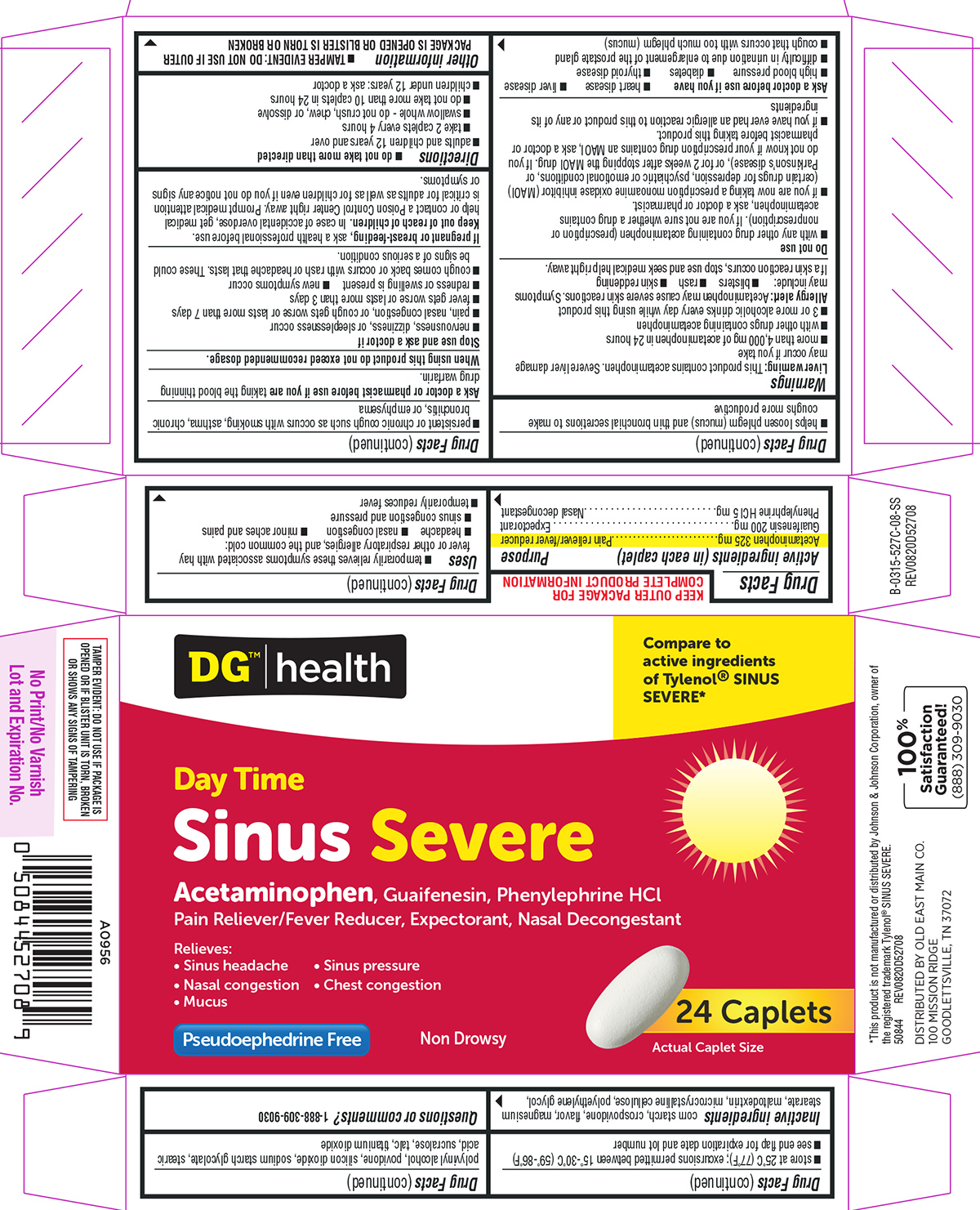
Label: SINUS SEVERE DAYTIME- acetaminophen, guaifenesin, phenylephrine hcl tablet, film coated
- NDC Code(s): 55910-529-08
- Packager: DOLGENCORP, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 25, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredients (in each caplet)
Acetaminophen 325 mg - Guaifenesin 200 mg - Phenylephrine HCl 5 mg
-
Purpose
Pain reliever/fever reducer - Expectorant - Nasal decongestant
-
Uses
temporarily relieves these symptoms associated with hay fever or other respiratory allergies, and the common cold: headache - nasal congestion - minor aches and pains - sinus congestion and ...
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take - more than 4,000 mg of acetaminophen in 24 hours - with other drugs containing acetaminophen - 3 or more ...
-
Directions
do not take more than directed - adults and children 12 years and over - take 2 caplets every 4 hours - swallow whole - do not crush, chew, or dissolve - do not take more than 10 caplets in 24 ...
-
Other information
each caplet contains: sodium 3 mg - TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN - store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F) see ...
-
Inactive ingredients
corn starch, crospovidone, flavor, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, silicon dioxide, sodium starch glycolate, stearic ...
-
Questions or comments?
1-888-309-9030
-
Principal Display Panel
DG®|health - Compare to - active ingredients - of Tylenol® SINUS - SEVERE* Day Time - Sinus Severe - Acetaminophen, Guaifenesin, Phenylephrine HCl - Pain Reliever/Fever Reducer, Expectorant, Nasal ...
-
INGREDIENTS AND APPEARANCEProduct Information