Label: BUSPIRONE HYDROCHLORIDE tablet
- NDC Code(s): 71335-1673-1, 71335-1673-2, 71335-1673-3, 71335-1673-4, view more
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 29300-245
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
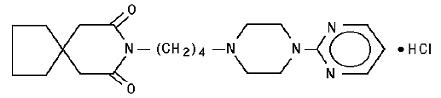
DESCRIPTIONBuspirone hydrochloride tablets, USP are an antianxiety agent that is not chemically or pharmacologically related to the benzodiazepines, barbiturates, or other sedative/anxiolytic ...
-
CLINICAL PHARMACOLOGYThe mechanism of action of buspirone is unknown. Buspirone differs from typical benzodiazepine anxiolytics in that it does not exert anticonvulsant or muscle relaxant effects. It also lacks the ...
-
INDICATIONS AND USAGEBuspirone hydrochloride tablets, USP are indicated for the management of anxiety disorders or the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of ...
-
CONTRAINDICATIONSBuspirone hydrochloride tablets are contraindicated in patients hypersensitive to buspirone hydrochloride. The use of monoamine oxidase inhibitors (MAOIs) intended to treat depression with ...
-
WARNINGSThe administration of buspirone hydrochloride to a patient taking a monoamine oxidase inhibitor (MAOI) may pose a hazard. There have been reports of the occurrence of elevated blood pressure when ...
-
PRECAUTIONSGeneral - Interference with Cognitive and Motor Performance - Studies indicate that buspirone hydrochloride is less sedating than other anxiolytics and that it does not produce significant ...
-
ADVERSE REACTIONS(See also PRECAUTIONS) Commonly Observed - The more commonly observed untoward events associated with the use of buspirone hydrochloride not seen at an equivalent incidence among ...
-
DRUG ABUSE AND DEPENDENCEControlled Substance Class - Buspirone hydrochloride is not a controlled substance. Physical and Psychological Dependence - In human and animal studies, buspirone has shown no potential for ...
-
OVERDOSAGESigns and Symptoms - In clinical pharmacology trials, doses as high as 375 mg/day were administered to healthy male volunteers. As this dose was approached, the following symptoms were observed ...
-
DOSAGE AND ADMINISTRATIONThe recommended initial dose is 15 mg daily (7.5 mg b.i.d.). To achieve an optimal therapeutic response, at intervals of 2 to 3 days the dosage may be increased 5 mg per day, as needed. The ...
-
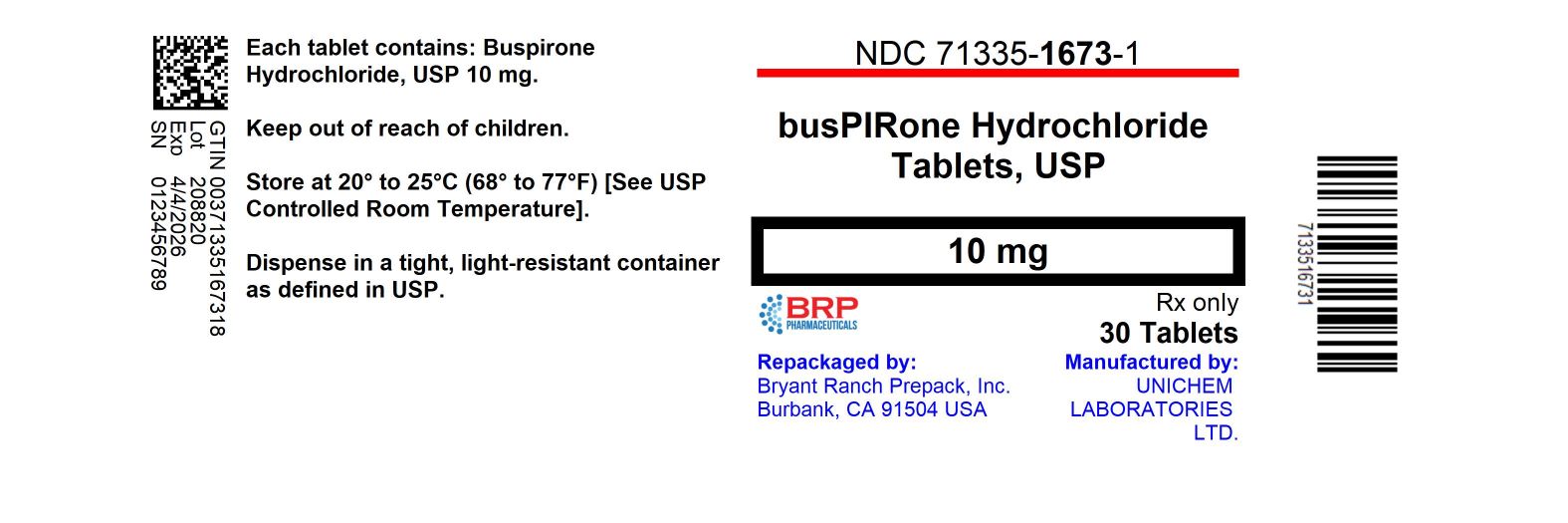
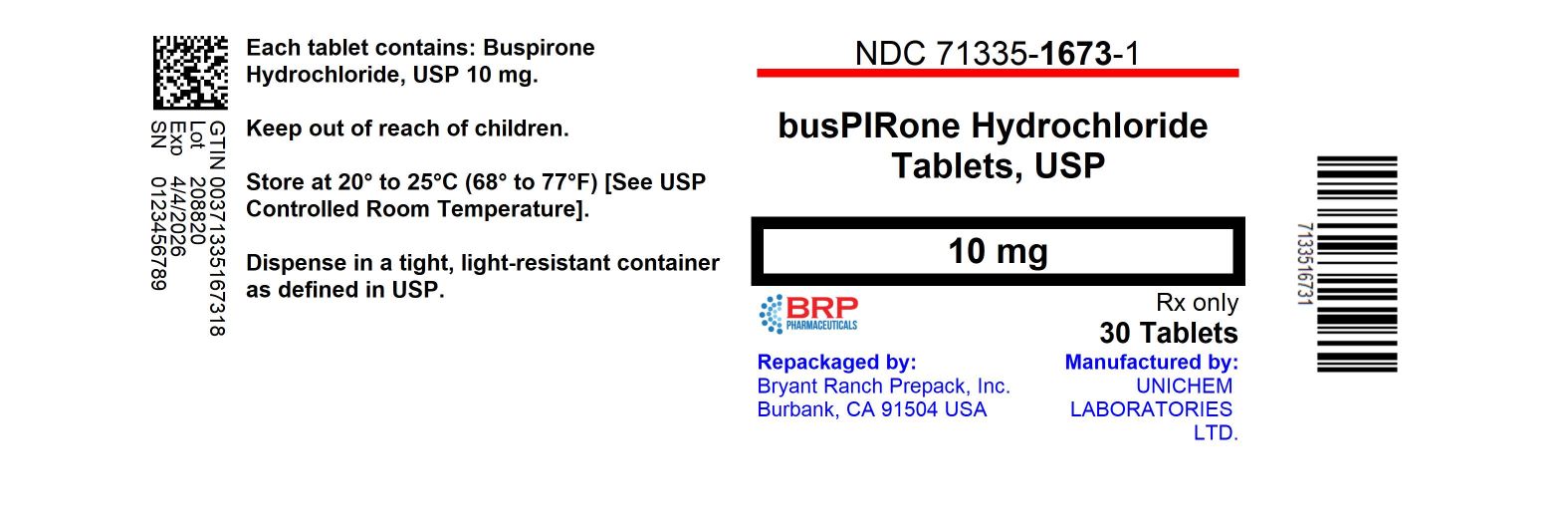
HOW SUPPLIEDWhite to off white, round flat faced beveled edge tablet, debossed with "245" on one side and "U" on either side of the break line. Product: 71335-1673 - NDC: 71335-1673-1 30 TABLET in a BOTTLE - NDC ...
-
REFERENCESAmerican Psychiatric Association, Ed.: Diagnostic and Statistical Manual of Mental Disorders—III, American Psychiatric Association, May 1980. Manufactured by: UNICHEM LABORATORIES LTD. Ind ...
-
PATIENT MEDICATION INFORMATION SECTIONBuspirone Hydrochloride Tablets, USP - Rx Only - HOW TO USE: Buspirone Hydrochloride Tablets, USP, 15 mg and 30 mg - Response to buspirone varies among individuals. Your physician may find it ...
-
PRINCIPAL DISPLAY PANELBuspirone Hcl 10mg Tablet
-
INGREDIENTS AND APPEARANCEProduct Information