Label: GALLIUM GA-68 PSMA-11- gallium ga-68 gozetotide injection, solution
- NDC Code(s): 24275-0525-1
- Packager: UCSF Radiopharmaceutical Facility
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GALLIUM GA 68 GOZETOTIDE - § Injection safely and effectively. See full prescribing information for GALLIUM GA 68 GOZETOTIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEGallium Ga 68 Gozeotide Injection is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer: with suspected ...
-
2 DOSAGE AND ADMINISTRATION2.1 Radiation Safety – Drug Handling - Handle Gallium Ga 68 Gozetotide Injection with appropriate safety measures to minimize radiation exposure - [see - Warnings and ...
-
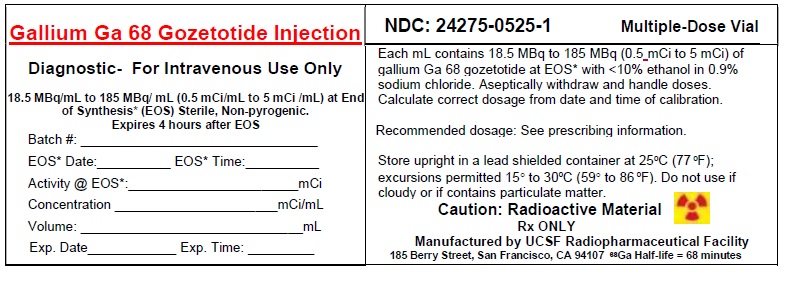
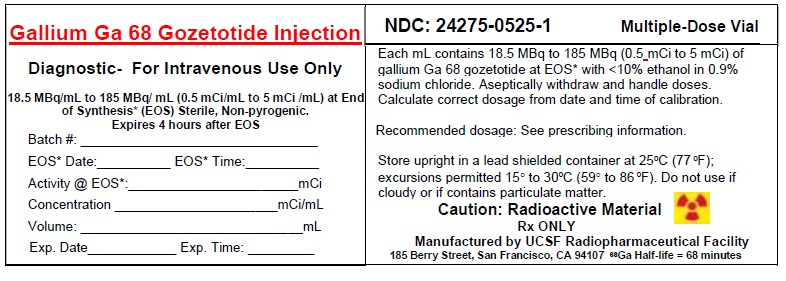
3 DOSAGE FORMS AND STRENGTHSInjection: supplied as a clear, colorless solution in a 20 mL multiple-dose vial containing 18.5 MBq/mL to 185 MBq/mL (0.5 mCi/mL to 5 mCi/mL) of Gallium Ga 68 Gozetotide at calibration ...
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Risk for Misdiagnosis - Image interpretation errors can occur with gallium Ga 68 gozetotide PET. A negative image does not rule out the presence of prostate cancer and a positive image does ...
-
6 ADVERSE REACTIONSClinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared ...
-
7 DRUG INTERACTIONSAndrogen deprivation therapy and other therapies targeting the androgen pathway - Androgen deprivation therapy (ADT) and other therapies targeting the androgen pathway, such as androgen ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Gallium Ga 68 Gozetotide Injection is not indicated for use in females. There are no available data with Gallium Ga 68 Gozetotide Injection use in pregnant women ...
-
10 OVERDOSAGEIn the event of an overdose of Gallium Ga 68 Gozetotide Injection, reduce the radiation absorbed dose to the patient where possible by increasing the elimination of the drug from the body using ...
-
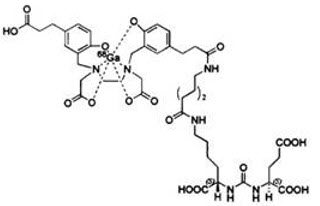
11 DESCRIPTION11.1 Chemical Characteristics - Gallium Ga Gozetotide Injection is a radioactive diagnostic agent for intravenous administration. It contains 0.5 mcg/mL Gozetotide, 18.5 MBq/mL to 185 MBq/mL (0.5 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Gallium Ga 68 gozetotode binds to prostate-specific membrane antigen (PSMA). It binds to cells that express PSMA, including malignant prostate cancer cells, which ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No long-term animal studies were performed to evaluate the carcinogenicity potential of gallium Ga 68 gozetotide.
-
14 CLINICAL STUDIESThe safety and efficacy of Gallium Ga 68 Gozetotide Injection were established in two prospective, open-label - studies, PSMA-PreRP (NCT03368547 and NCT02919111) and PSMA-BCR ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Gallium Ga 68 Gozetotide Injection (NDC 24275-0525-1) is a clear, colorless solution, supplied in a capped glass vial containing 18.5 MBq/mL to 185 MBq/mL (0.5 mCi/mL to 5 ...
-
17 PATIENT COUNSELING INFORMATIONAdequate Hydration - Instruct patients to drink a sufficient amount of water to ensure adequate hydration before their PET study and urge them to drink and urinate as often as possible during ...
-
SPL UNCLASSIFIED SECTIONManufactured and Distributed by: University of California, San Francisco - UCSF Radiopharmaceutical Facility - 185 Berry Street - Suite 350 - San ...
-
Drug Product Label Vial and Radiation Shield
-
INGREDIENTS AND APPEARANCEProduct Information