Label: BIO-MYCIN 200- oxytetracycline injection
- NDC Code(s): 0010-4753-01, 0010-4753-02, 0010-4753-03
- Packager: Boehringer Ingelheim Animal Health USA Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated March 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONApproved by FDA under ANADA # 200-008 - Antibiotic - Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
-
DESCRIPTIONEach mL contains 200 mg oxytetracycline. For the treatment of disease in beef cattle, dairy cattle and swine. For animal use only - Read entire insert carefully before using this product. BIO-MYCIN ...
-
Caution: When administered to cattle, muscle discoloration may necessitate trimming of the injection site(s) and surrounding tissues during the dressing procedure.
-
Warning: Discontinue treatment at least 28 days prior to slaughter of cattle and swine. Milk taken from animals during treatment and for 96 hours after the last treatment must not be used for food. Rapid ...
-
Precautions: Exceeding the highest recommended dosage level of drug per pound of body weight per day, administering more than the recommended number of treatments and/or exceeding 10 mL intramuscularly or ...
-
Adverse Reactions: Reports of adverse reactions associated with oxytetracycline administration include injection site swelling, restlessness, ataxia, trembling, swelling of eyelids, ears, muzzle, anus and vulva (or ...
-
Storage: Store at controlled room temperature 15°-25°C (59°-77°F). Exposure to colder temperatures may cause cloudiness. Gently warm the product to restore clarity. Use within 12 months of first puncture ...
-
Care of Sick Animals: The use of antibiotics in the management of diseases is based on an accurate diagnosis and an adequate course of treatment. When properly used in the treatment of diseases caused by ...
-
Indications: BIO-MYCIN 200 is intended for use in the treatment of the following diseases in beef cattle, dairy cattle and swine when due to oxytetracycline-susceptible organisms: Cattle: In cattle, BIO-MYCIN ...
-
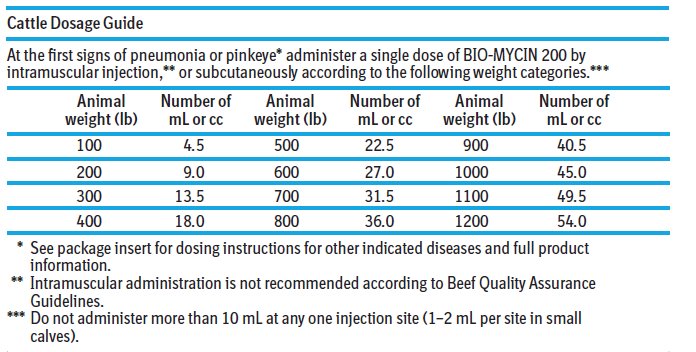
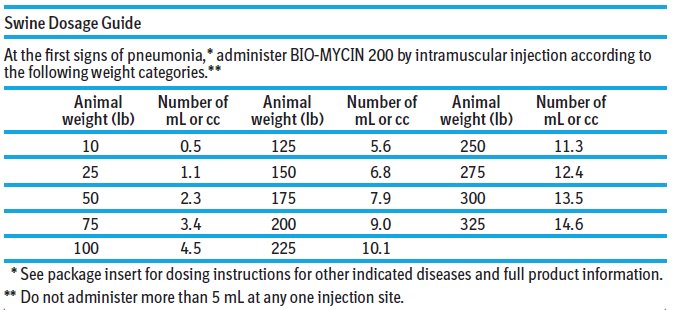
Dosage: Cattle: BIO-MYCIN 200 is to be administered by intramuscular, subcutaneous, or intravenous injection. Intramuscular administration is not recommended according to Beef Quality Assurance ...
-
Directions for Use: BIO-MYCIN 200 is intended for use in the treatment of disease due to oxytetracycline susceptible organisms in beef cattle, dairy cattle and swine. A thoroughly cleaned, sterile needle and syringe ...
-
Intramuscular Administration: Intramuscular injections should be made by directing the needle of suitable gauge and length into the fleshy part of a thick muscle in the neck region; avoid blood vessels and major nerves. Before ...
-
Subcutaneous Administration: Subcutaneous injections in beef cattle and dairy cattle should be made by directing the needle of suitable gauge and length through the loose folds of the neck skin in front of the shoulder. Care ...
-
Intravenous Administration: BIO-MYCIN 200 (oxytetracycline injection) may be administered intravenously to beef cattle and dairy cattle. As with all highly concentrated materials, BIO-MYCIN 200 should be administered slowly ...
-
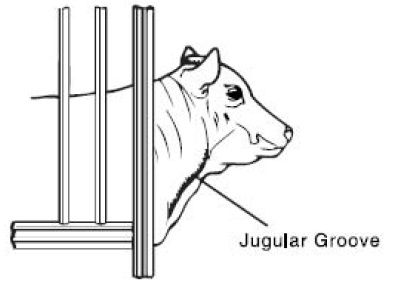
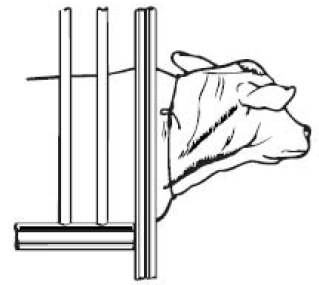
Preparation of the Animal for Injection: 1. Approximate location of vein. The jugular vein runs in the jugular groove on each side of the neck from the angle of the jaw to just above the brisket and slightly above and to the side of the ...
-
Entering the Vein and Making the Injection: 1. Raise the vein. This is accomplished by tying the choke rope tightly around the neck close to the shoulder. The rope should be tied in such a way that it will not come loose and so that it can ...
-
How Supplied: BIO-MYCIN 200 is available in 100-mL, 250-mL, and 500-mL bottles containing 200 mg oxytetracycline per mL - NDC 0010-4753-01 - 100 mL - NDC 0010-4753-02 - 250 mL - NDC 0010-4753-03 - 500 mL
-
SPL UNCLASSIFIED SECTIONNot For Human Use. BIO-MYCIN is a registered trademark of Boehringer Ingelheim Animal Health USA Inc. © 2023 Boehringer Ingelheim Animal Health USA Inc. All rights ...
-
Principal Display Panel – Container Label 500 mL NDC 0010-4753-03 - Bio-Mycin® 200 - (oxytetracycline injection) Antibiotic - Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Each mL contains 200 mg ...
-
INGREDIENTS AND APPEARANCEProduct Information