Label: SULFAMETHOXAZOLE AND TRIMETHOPRIM suspension
- NDC Code(s): 62135-749-47, 62135-873-24, 62135-873-52
- Packager: Chartwell RX, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of sulfamethoxazole and trimethoprim oral suspension and other antibacterial drugs, sulfamethoxazole and ...
To reduce the development of drug-resistant bacteria and maintain the effectiveness of sulfamethoxazole and trimethoprim oral suspension and other antibacterial drugs, sulfamethoxazole and trimethoprim oral suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Close -
DESCRIPTIONSulfamethoxazole and Trimethoprim Oral Suspension, USP is a synthetic antibacterial combination product with each teaspoonful (5 mL) containing 200 mg sulfamethoxazole and 40 mg ...
Sulfamethoxazole and Trimethoprim Oral Suspension, USP is a synthetic antibacterial combination product with each teaspoonful (5 mL) containing 200 mg sulfamethoxazole and 40 mg trimethoprim.
Sulfamethoxazole is N1-(5-methyl-3-isoxazolyl) sulfanilamide; the molecular formula is C 10H 11N 3O 3S. It is an almost white, odorless, tasteless compound with a molecular weight of 253.28 and the following structural formula:
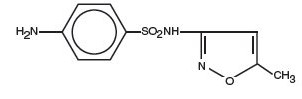
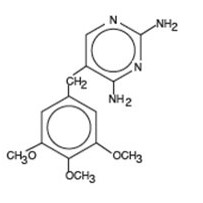
Trimethoprim is 2,4-diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine; the molecular formula is C 14H 18N 4O 3. It is a white to light yellow, odorless, bitter compound with a molecular weight of 290.3 and the following structural formula:
Each teaspoonful (5 mL) of sulfamethoxazole and trimethoprim oral suspension (cherry flavor) is an opaque pinkish orange suspension with a cherry aroma and contains 40 mg trimethoprim and 200 mg sulfamethoxazole and the inactive ingredients alcohol not more than 0.5%; methylparaben 0.1% and sodium benzoate 0.1% (added as preservatives), artificial wild cherry flavor, citric acid anhydrous, FD&C Red #40, FD&C Yellow #6, glycerin, microcrystalline cellulose, polysorbate 80, purified water, saccharin sodium, sodium carboxymethylcellulose, sodium citrate dihydrate, and sorbitol solution.
Close -
CLINICAL PHARMACOLOGYSulfamethoxazole and trimethoprim is rapidly absorbed following oral administration. Both sulfamethoxazole and trimethoprim exist in the blood as unbound, protein-bound and metabolized forms ...
Sulfamethoxazole and trimethoprim is rapidly absorbed following oral administration. Both sulfamethoxazole and trimethoprim exist in the blood as unbound, protein-bound and metabolized forms; sulfamethoxazole also exists as the conjugated form. Sulfamethoxazole is metabolized in humans to at least 5 metabolites: the N 4-acetyl-, N 4-hydroxy-, 5-methylhydroxy-, N 4-acetyl-5-methylhydroxy- sulfamethoxazole metabolites, and an N-glucuronide conjugate. The formation of N 4-hydroxy metabolite is mediated via CYP2C9.
Trimethoprim is metabolized in vitroto 11 different metabolites, of which, five are glutathione adducts and six are oxidative metabolites, including the major metabolites, 1- and 3-oxides and the 3- and 4-hydroxy derivatives.
The free forms of sulfamethoxazole and trimethoprim are considered to be the therapeutically active forms.
In vitro studies suggest that trimethoprim is a substrate of P-glycoprotein, OCT1 and OCT2, and that sulfamethoxazole is not a substrate of P-glycoprotein.
Approximately 70% of sulfamethoxazole and 44% of trimethoprim are bound to plasma proteins. The presence of 10 mg percent sulfamethoxazole in plasma decreases the protein binding of trimethoprim by an insignificant degree; trimethoprim does not influence the protein binding of sulfamethoxazole.
Peak blood levels for the individual components occur 1 to 4 hours after oral administration. The mean serum half-lives of sulfamethoxazole and trimethoprim are 10 and 8 to 10 hours, respectively. However, patients with severely impaired renal function exhibit an increase in the half-lives of both components, requiring dosage regimen adjustment (see DOSAGE AND ADMINISTRATION). Detectable amounts of sulfamethoxazole and trimethoprim are present in the blood 24 hours after drug administration. During administration of 800 mg sulfamethoxazole and 160 mg trimethoprim b.i.d., the mean steady-state plasma concentration of trimethoprim was 1.72 mcg/mL. The steady-state mean plasma levels of free and total sulfamethoxazole were 57.4 mcg/mL and 68 mcg/mL, respectively. These steady-state levels were achieved after three days of drug administration. 1 Excretion of sulfamethoxazole and trimethoprim is primarily by the kidneys through both glomerular filtration and tubular secretion. Urine concentrations of both sulfamethoxazole and trimethoprim are considerably higher than are the concentrations in the blood. The average percentage of the dose recovered in urine from 0 to 72 hours after a single oral dose of sulfamethoxazole and trimethoprim is 84.5% for total sulfonamide and 66.8% for free trimethoprim. Thirty percent of the total sulfonamide is excreted as free sulfamethoxazole, with the remaining as N 4-acetylated metabolite. 2 When administered together as sulfamethoxazole and trimethoprim, neither sulfamethoxazole nor trimethoprim affects the urinary excretion pattern of the other.
Both sulfamethoxazole and trimethoprim distribute to sputum, vaginal fluid and middle ear fluid; trimethoprim also distributes to bronchial secretion, and both pass the placental barrier and are excreted in human milk.
Pharmacokinetics in Pediatric Patients
A simulation conducted with data from a pharmacokinetic study in 153 infants and children demonstrated that mean steady state AUC and maximum plasma concentration of trimethoprim and sulfamethoxazole would be comparable between pediatric patients 2 months to 18 years receiving 8/40 (trimethoprim/ sulfamethoxazole) mg/kg/day divided every 12 hours and adult patients receiving 320/1600 (trimethoprim/sulfamethoxazole) mg/day.
Pharmacokinetics in Geriatric Patients
The pharmacokinetics of sulfamethoxazole 800 mg and trimethoprim 160 mg were studied in 6 geriatric subjects (mean age: 78.6 years) and 6 young healthy subjects (mean age: 29.3 years) using a non-US approved formulation. Pharmacokinetic values for sulfamethoxazole in geriatric subjects were similar to those observed in young adult subjects. The mean renal clearance of trimethoprim was significantly lower in geriatric subjects compared with young adult subjects (19 mL/h/kg vs. 55 mL/h/kg). However, after normalizing by body weight, the apparent total body clearance of trimethoprim was on average 19% lower in geriatric subjects compared with young adult subjects. 3
Microbiology
Mechanism of Action
Sulfamethoxazole inhibits bacterial synthesis of dihydrofolic acid by competing with para-aminobenzoic acid (PABA). Trimethoprim blocks the production of tetrahydrofolic acid from dihydrofolic acid by binding to and reversibly inhibiting the required enzyme, dihydrofolate reductase. Thus, sulfamethoxazole and trimethoprim blocks two consecutive steps in the biosynthesis of nucleic acids and proteins essential to many bacteria.
Resistance
In vitro studies have shown that bacterial resistance develops more slowly with both sulfamethoxazole and trimethoprim in combination than with either sulfamethoxazole or trimethoprim alone.
Antimicrobial Activity
Sulfamethoxazole and trimethoprim has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGEsection.
Aerobic gram-positive bacteria
Streptococcus pneumoniae
Aerobic gram-negative bacteria
Escherichia coli (including susceptible enterotoxigenic strains implicated in traveler’s diarrhea)
Klebsiella species
Enterobacter species
Haemophilus influenzae
Morganella morganii
Proteus mirabilis
Proteus vulgaris
Shigella flexneri
Shigella sonnei
Other Microorganisms
Pneumocystis jirovecii
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
Close -
INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of sulfamethoxazole and trimethoprim oral suspension and other antibacterial drugs, sulfamethoxazole and ...
To reduce the development of drug-resistant bacteria and maintain the effectiveness of sulfamethoxazole and trimethoprim oral suspension and other antibacterial drugs, sulfamethoxazole and trimethoprim oral suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to empiric selection of therapy.
Urinary Tract Infections
For the treatment of urinary tract infections due to susceptible strains of the following organisms: Escherichia coli, Klebsiella species, Enterobacter species, Morganella morganii, Proteus mirabilisand Proteus vulgaris. It is recommended that initial episodes of uncomplicated urinary tract infections be treated with a single effective antibacterial agent rather than the combination.
Acute Otitis Media
For the treatment of acute otitis media in pediatric patients due to susceptible strains of Streptococcus pneumoniaeor Haemophilus influenzaewhen in the judgment of the physician sulfamethoxazole and trimethoprim offers some advantage over the use of other antimicrobial agents. To date, there are limited data on the safety of repeated use of sulfamethoxazole and trimethoprim in pediatric patients under two years of age. Sulfamethoxazole and trimethoprim is not indicated for prophylactic or prolonged administration in otitis media at any age.
Acute Exacerbations of Chronic Bronchitis in Adults
For the treatment of acute exacerbations of chronic bronchitis due to susceptible strains of Streptococcus pneumoniaeor Haemophilus influenzaewhen a physician deems that sulfamethoxazole and trimethoprim could offer some advantage over the use of a single antimicrobial agent.
Shigellosis
For the treatment of enteritis caused by susceptible strains of Shigella flexneriand Shigella sonnei when antibacterial therapy is indicated.
Pneumocystis jirovecii Pneumonia
For the treatment of documented Pneumocystis jiroveciipneumonia and for prophylaxis against P. jiroveciipneumonia in individuals who are immunosuppressed and considered to be at an increased risk of developing P. jirovecii pneumonia.
Traveler’s Diarrhea in Adults
For the treatment of traveler’s diarrhea due to susceptible strains of enterotoxigenic E. coli.
Close -
CONTRAINDICATIONSSulfamethoxazole and trimethoprim is contraindicated in the following situations: known hypersensitivity to trimethoprim or sulfonamides - history of drug-induced immune thrombocytopenia with use ...
Sulfamethoxazole and trimethoprim is contraindicated in the following situations:
- known hypersensitivity to trimethoprim or sulfonamides
- history of drug-induced immune thrombocytopenia with use of trimethoprim and/or sulfonamides
- documented megaloblastic anemia due to folate deficiency
- pediatric patients less than 2 months of age
- marked hepatic damage
- severe renal insufficiency when renal function status cannot be monitored
- concomitant administration with dofetilide (see PRECAUTIONS).
-
WARNINGSEmbryofetal Toxicity - Some epidemiologic studies suggest that exposure to sulfamethoxazole and trimethoprim during pregnancy may be associated with an increased risk of congenital malformations ...
Embryofetal Toxicity
Some epidemiologic studies suggest that exposure to sulfamethoxazole and trimethoprim during pregnancy may be associated with an increased risk of congenital malformations, particularly neural tube defects, cardiovascular malformations, urinary tract defects, oral clefts, and club foot. If sulfamethoxazole and trimethoprim is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be advised of the potential hazards to the fetus (see PRECAUTIONS).
Hypersensitivity and Other Serious or Fatal Reactions
Fatalities and serious adverse reactions including severe cutaneous adverse reactions (SCARs) including Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS), acute febrile neutrophilic dermatosis (AFND), acute generalized erythematous pustulosis (AGEP); fulminant hepatic necrosis; agranulocytosis, aplastic anemia and other blood dyscrasias; acute and delayed lung injury; anaphylaxis and circulatory shock have occurred with the administration of sulfamethoxazole and trimethoprim products, including sulfamethoxazole and trimethoprim oral suspension (see ADVERSE REACTIONS).
Hypersensitivity Reactions of the Respiratory Tract
Cough, shortness of breath and pulmonary infiltrates potentially representing hypersensitivity reactions of the respiratory tract have been reported in association with sulfamethoxazole and trimethoprim treatment.
Respiratory Failure
Other severe pulmonary adverse reactions occurring within days to week of sulfamethoxazole and trimethoprim initiation and resulting in prolonged respiratory failure requiring mechanical ventilation or extracorporeal membrane oxygenation (ECMO), lung transplantation or death have also been reported in patients and otherwise healthy individuals treated with sulfamethoxazole and trimethoprim products
Circulatory Shock
Circulatory shock with fever, severe hypotension, and confusion requiring intravenous fluid resuscitation and vasopressors has occurred within minutes to hours of re-challenge with sulfamethoxazole and trimethoprim products, including sulfamethoxazole and trimethoprim, in patients with history of recent (days to weeks) exposure to sulfamethoxazole and trimethoprim.
Management of Hypersensitivity and Other Serious Reactions
Sulfamethoxazole and trimethoprim should be discontinued at the first appearance of skin rash or any sign of a serious adverse reaction. A skin rash may be followed by a more severe reaction, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, DRESS, AFND, AGEP, hepatic necrosis, or serious blood disorders (see PRECAUTIONSand ADVERSE REACTIONS). Clinical signs, such as rash, pharyngitis, fever, arthralgia, cough, chest pain, dyspnea, pallor, purpura or jaundice may be early indications of serious reactions.
Hemophagocytic Lymphohistiocytosis
Cases of hemophagocytic lymphohistiocytosis (HLH) have been reported in patients treated with sulfamethoxazole-trimethoprim. HLH is a lifethreatening syndrome of pathologic immune activation characterized by clinical signs and symptoms of extreme systemic inflammation. Signs and symptoms of HLH may include fever, hepatosplenomegaly, rash, lymphadenopathy, neurologic symptoms, cytopenias, high serum ferritin, hypertriglyceridemia, and liver enzyme and coagulation abnormalities. If HLH is suspected, discontinue Sulfamethoxazole and trimethoprim oral suspension immediately and institute appropriate management.
Thrombocytopenia
Sulfamethoxazole and trimethoprim-induced thrombocytopenia may be an immune-mediated disorder. Severe cases of thrombocytopenia that are fatal or life threatening have been reported. Thrombocytopenia usually resolves within a week upon discontinuation of sulfamethoxazole and trimethoprim.
Streptococcal Infections and Rheumatic Fever
The sulfonamides should not be used for treatment of group A β-hemolytic streptococcal infections. In an established infection, they will not eradicate the streptococcus and, therefore, will not prevent sequelae such as rheumatic fever.
Clostridioides difficile Associated Diarrhea
Clostridioides difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including sulfamethoxazole and trimethoprim, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Risk Associated with Concurrent Use of Leucovorin for Pneumocystis jirovecii Pneumonia
Treatment failure and excess mortality were observed when sulfamethoxazole and trimethoprim was used concomitantly with leucovorin for the treatment of HIV positive patients with P. jiroveciipneumonia in a randomized placebo-controlled trial. 4Avoid coadministration of sulfamethoxazole and trimethoprim and leucovorin during treatment of P. jirovecii pneumonia.
Close -
PRECAUTIONSDevelopment of Drug Resistant Bacteria - Prescribing sulfamethoxazole and trimethoprim oral suspension in the absence of a proven or strongly suspected bacterial infection or a prophylactic ...
Development of Drug Resistant Bacteria
Prescribing sulfamethoxazole and trimethoprim oral suspension in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Folate Deficiency
Avoid use of sulfamethoxazole and trimethoprim in patients with impaired renal or hepatic function, in those with possible folate deficiency (e.g., the elderly, chronic alcoholics, patients receiving anticonvulsant therapy, patients with malabsorption syndrome, and patients in malnutrition states) and in those with severe allergies or bronchial asthma.
Hematological changes indicative of folic acid deficiency may occur in elderly patients or in patients with preexisting folic acid deficiency or kidney failure. These effects are reversible by folinic acid therapy (see PRECAUTIONS, Geriatric Use).
Hemolysis
In glucose-6-phosphate dehydrogenase deficient individuals, hemolysis may occur. This reaction is frequently dose-related.
Hypoglycemia
Cases of hypoglycemia in non-diabetic patients treated with sulfamethoxazole and trimethoprim are seen rarely, usually occurring after a few days of therapy. Patients with renal dysfunction, liver disease, malnutrition or those receiving high doses of sulfamethoxazole and trimethoprim are particularly at risk.
Impaired Phenylalanine Metabolism
The trimethoprim component of sulfamethoxazole and trimethoprim has been noted to impair phenylalanine metabolism, but this is of no significance in phenylketonuric patients on appropriate dietary restriction.
Porphyria and Hypothyroidism
Like other drugs containing sulfonamides, sulfamethoxazole and trimethoprim can precipitate porphyria crisis and hypothyroidism. Avoid use of sulfamethoxazole and trimethoprim in patients with porphyria or thyroid dysfunction.
Potential Risk in the Treatment of Pneumocystis jirovecii Pneumonia in Patients with Acquired Immunodeficiency Syndrome (AIDS)
AIDS patients may not tolerate or respond to sulfamethoxazole and trimethoprim in the same manner as non-AIDS patients. The incidence of adverse reactions, particularly rash, fever, leukopenia and elevated aminotransferase (transaminase) values, with sulfamethoxazole and trimethoprim therapy in AIDS patients who are being treated for P. jirovecii pneumonia has been reported to be increased compared with the incidence normally associated with the use of sulfamethoxazole and trimethoprim in non-AIDS patients. If a patient develops skin rash, fever, leukopenia or any sign of adverse reaction, reevaluate benefit-risk of continuing therapy or re-challenge with sulfamethoxazole and trimethoprim (see WARNINGS).
Avoid coadministration of sulfamethoxazole and trimethoprim and leucovorin during treatment of P. jirovecii pneumonia (see WARNINGS).
Electrolyte Abnormalities
Hyperkalemia: High dosage of trimethoprim, as used in patients with P. jirovecii pneumonia, induces a progressive but reversible increase of serum potassium concentrations in a substantial number of patients. Even treatment with recommended doses may cause hyperkalemia when trimethoprim is administered to patients with underlying disorders of potassium metabolism, with renal insufficiency, or if drugs known to induce hyperkalemia are given concomitantly. Close monitoring of serum potassium is warranted in these patients.
Hyponatremia: Severe and symptomatic hyponatremia can occur in patients receiving sulfamethoxazole and trimethoprim, particularly for the treatment of P. jirovecii pneumonia. Evaluation for hyponatremia and appropriate correction is necessary in symptomatic patients to prevent life-threatening complications.
Crystalluria: During treatment, ensure adequate fluid intake and urinary output to prevent crystalluria. Patients who are “slow acetylators” may be more prone to idiosyncratic reactions to sulfonamides.
Information for Patients
Hypersensitivity and Other Serious Reactions: Advise patients to stop taking sulfamethoxazole and trimethoprim oral suspension immediately if they experience any clinical signs such as rash, pharyngitis, fever, arthralgia, cough, chest pain, dyspnea, pallor, purpura or jaundice and to contact their healthcare provider as soon as possible (see WARNINGSand ADVERSE REACTIONS).
Antibacterial Resistance:Patients should be counseled that antibacterial drugs including sulfamethoxazole and trimethoprim oral suspension should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When sulfamethoxazole and trimethoprim oral suspension is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by sulfamethoxazole and trimethoprim oral suspension or other antibacterial drugs in the future.
Crystalluria and Stone Formation:Advise patients to maintain an adequate fluid intake in order to prevent crystalluria and stone formation.
Diarrhea:Advise patients that diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic.If this occurs, patients should contact their physician as soon as possible.
Laboratory Tests
Complete blood counts and clinical chemistry testing should be done frequently in patients receiving sulfamethoxazole and trimethoprim. Perform urinalyses with careful microscopic examination and renal function tests during therapy, particularly for those patients with impaired renal function. Discontinue sulfamethoxazole and trimethoprim if a significant electrolyte abnormality, renal insufficiency or reduction in the count of any formed blood element is noted.
Drug Interactions
Potential for sulfamethoxazole and trimethoprim to Affect Other Drugs
Trimethoprim is an inhibitor of CYP2C8 as well as OCT2 transporter. Sulfamethoxazole is an inhibitor of CYP2C9. Avoid coadministration of sulfamethoxazole and trimethoprim with drugs that are substrates of CYP2C8 and 2C9 or OCT2.
Table 1: Drug Interactions with Sulfamethoxazole and Trimethoprim
Drug(s)
Recommendation
Comments
Diuretics
Avoid concurrent use
In elderly patients concurrently receiving certain diuretics, primarily thiazides, an increased incidence of thrombocytopenia with purpura has been reported.
Warfarin
Monitor prothrombin time and INR
It has been reported that sulfamethoxazole and trimethoprim may prolong the prothrombin time in patients who are receiving the anticoagulant warfarin (a CYP2C9 substrate). This interaction should be kept in mind when sulfamethoxazole and trimethoprim is given to patients already on anticoagulant therapy, and the coagulation time should be reassessed.
Phenytoin
Monitor serum phenytoin levels
Sulfamethoxazole and trimethoprim may inhibit the hepatic metabolism of phenytoin (a CYP2C9 substrate). Sulfamethoxazole and trimethoprim, given at a common clinical dosage, increased the phenytoin half-life by 39% and decreased the phenytoin metabolic clearance rate by 27%. When administering these drugs concurrently, one should be alert for possible excessive phenytoin effect.
Methotrexate
Avoid concurrent use
Sulfonamides can also displace methotrexate from plasma protein binding sites and can compete with the renal transport of methotrexate, thus increasing free methotrexate concentrations.
Cyclosporine
Avoid concurrent use
There have been reports of marked but reversible nephrotoxicity with coadministration of sulfamethoxazole and trimethoprim and cyclosporine in renal transplant recipients.
Digoxin
Monitor serum digoxin levels
Increased digoxin blood levels can occur with concomitant sulfamethoxazole and trimethoprim therapy, especially in elderly patients.
Indomethacin
Avoid concurrent use
Increased sulfamethoxazole blood levels may occur in patients who are also receiving indomethacin.
Pyrimethamine
Avoid concurrent use
Occasional reports suggest that patients receiving pyrimethamine as malaria prophylaxis in doses exceeding 25 mg weekly may develop megaloblastic anemia if sulfamethoxazole and trimethoprim is prescribed.
Tricyclic Antidepressants (TCAs)
Monitor therapeutic response and adjust dose of TCA accordingly
The efficacy of tricyclic antidepressants can decrease when coadministered with sulfamethoxazole and trimethoprim.
Oral Hypoglycemics
Monitor blood glucose more frequently
Like other sulfonamide-containing drugs, sulfamethoxazole and trimethoprim potentiates the effect of oral hypoglycemic that are metabolized by CYP2C8 (e.g., pioglitazone, repaglinide, and rosiglitazone) or CYP2C9 (e.g., glipizide and glyburide) or eliminated renally via OCT2 (e.g., metformin). Additional monitoring of blood glucose may be warranted.
Amantadine
Avoid concurrent use
In the literature, a single case of toxic delirium has been reported after concomitant intake of sulfamethoxazole and trimethoprim and amantadine (an OCT2 substrate). Cases of interactions with other OCT2 substrates, memantine and metformin, have also been reported.
Angiotensin Converting Enzyme Inhibitors
Avoid concurrent use
In the literature, three cases of hyperkalemia in elderly patients have been reported after concomitant intake of sulfamethoxazole and trimethoprim and an angiotensin converting enzyme inhibitor. 5,6
Zidovudine
Monitor for hematologic toxicity
Zidovudine and sulfamethoxazole and trimethoprim are known to induce hematological abnormalities. Hence, there is potential for an additive myelotoxicity when coadministered. 7
Dofetilide
Concurrent administration is contraindicated
Elevated plasma concentrations of dofetilide have been reported following concurrent administration of trimethoprim and dofetilide. Increased plasma concentrations of dofetilide may cause serious ventricular arrhythmias associated with QT interval prolongation, including torsade de pointes. 8,9
Procainamide
Closely monitor for clinical and ECG signs of procainamide toxicity and/or procainamide plasma concentration if available
Trimethoprim increases the plasma concentrations of procainamide and its active N-acetyl metabolite (NAPA) when trimethoprim and procainamide are coadministered. The increased procainamide and NAPA plasma concentrations that resulted from the pharmacokinetic interaction with trimethoprim are associated with further prolongation of the QTc interval. 10
Drug/Laboratory Test Interactions
Sulfamethoxazole and trimethoprim, specifically the trimethoprim component, can interfere with a serum methotrexate assay as determined by the competitive binding protein technique (CBPA) when a bacterial dihydrofolate reductase is used as the binding protein. No interference occurs, however, if methotrexate is measured by a radioimmunoassay (RIA).
The presence of sulfamethoxazole and trimethoprim may also interfere with the Jaffé alkaline picrate reaction assay for creatinine, resulting in overestimations of about 10% in the range of normal values.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Sulfamethoxazole was not carcinogenic when assessed in a 26-week tumorigenic mouse (Tg-rasH2) study at doses up to 400 mg/kg/day sulfamethoxazole; equivalent to 2.4-fold the human systemic exposure (at a daily dose of 800 mg sulfamethoxazole twice a day).
Mutagenesis
In vitro reverse mutation bacterial tests according to the standard protocol have not been performed with sulfamethoxazole and trimethoprim in combination. An in vitro chromosomal aberration test in human lymphocytes with sulfamethoxazole and trimethoprim was negative. In in vitroand in vivo tests in animal species, sulfamethoxazole and trimethoprim did not damage chromosomes. In vivo micronucleus assays were positive following oral administration of sulfamethoxazole and trimethoprim. Observations of leukocytes obtained from patients treated with sulfamethoxazole and trimethoprim revealed no chromosomal abnormalities.
Sulfamethoxazole alone was positive in an in vitro reverse mutation bacterial assay and in in vitro micronucleus assays using cultured human lymphocytes.
Trimethoprim alone was negative in in vitro reverse mutation bacterial assays and in in vitro chromosomal aberration assays with Chinese Hamster ovary or lung cells with or without S9 activation. In in vitro Comet, micronucleus and chromosomal damage assays using cultured human lymphocytes, trimethoprim was positive. In mice following oral administration of trimethoprim, no DNA damage in Comet assays of liver, kidney, lung, spleen, or bone marrow was recorded.
Impairment of Fertility
No adverse effects on fertility or general reproductive performance were observed in rats given oral dosages as high as 350 mg/kg/day sulfamethoxazole plus 70 mg/kg/day trimethoprim, doses roughly two times the recommended human daily dose on a body surface area basis.
Pregnancy
While there are no large, well-controlled studies on the use of sulfamethoxazole and trimethoprim in pregnant women, Brumfitt and Pursell, 11 in a retrospective study, reported the outcome of 186 pregnancies during which the mother received either placebo or sulfamethoxazole and trimethoprim. The incidence of congenital abnormalities was 4.5% (3 of 66) in those who received placebo and 3.3% (4 of 120) in those receiving sulfamethoxazole and trimethoprim. There were no abnormalities in the 10 children whose mothers received the drug during the first trimester. In a separate survey, Brumfitt and Pursell also found no congenital abnormalities in 35 children whose mothers had received oral sulfamethoxazole and trimethoprim at the time of conception or shortly thereafter.
Because sulfamethoxazole and trimethoprim may interfere with folic acid metabolism, sulfamethoxazole and trimethoprim should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Teratogenic Effects
Human Data
While there are no large prospective, well controlled studies in pregnant women and their babies, some retrospective epidemiologic studies suggest an association between first trimester exposure to sulfamethoxazole and trimethoprim with an increased risk of congenital malformations, particularly neural tube defects, cardiovascular abnormalities, urinary tract defects, oral clefts, and club foot. These studies, however, were limited by the small number of exposed cases and the lack of adjustment for multiple statistical comparisons and confounders. These studies are further limited by recall, selection, and information biases, and by limited generalizability of their findings. Lastly, outcome measures varied between studies, limiting cross-study comparisons. Alternatively, other epidemiologic studies did not detect statistically significant associations between sulfamethoxazole and trimethoprim exposure and specific malformations.
Animal Data
In rats, oral doses of either 533 mg/kg sulfamethoxazole or 200 mg/kg trimethoprim produced teratologic effects manifested mainly as cleft palates. These doses are approximately 5 and 6 times the recommended human total daily dose on a body surface area basis. In two studies in rats, no teratology was observed when 512 mg/kg of sulfamethoxazole was used in combination with 128 mg/kg of trimethoprim. In some rabbit studies, an overall increase in fetal loss (dead and resorbed conceptuses) was associated with doses of trimethoprim 6 times the human therapeutic dose based on body surface area.
Nonteratogenic Effects
See CONTRAINDICATIONSsection.
Nursing Mothers
Levels of sulfamethoxazole and trimethoprim in breast milk are approximately 2–5% of the recommended daily dose for infants over 2 months of age. Caution should be exercised when sulfamethoxazole and trimethoprim is administered to a nursing woman, especially when breastfeeding jaundiced, ill, stressed, or premature infants because of the potential risk of bilirubin displacement and kernicterus.
Pediatric Use
Sulfamethoxazole and trimethoprim is contraindicated for infants younger than 2 months of age (see INDICATIONS AND USAGEand CONTRAINDICATIONS).
Geriatric Use
Clinical studies of sulfamethoxazole and trimethoprim did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
There may be an increased risk of severe adverse reactions in elderly patients, particularly when complicating conditions exist, e.g., impaired kidney and/or liver function, possible folate deficiency, or concomitant use of other drugs. Severe skin reactions, generalized bone marrow suppression (see WARNINGSand ADVERSE REACTIONS), a specific decrease in platelets (with or without purpura), and hyperkalemia are the most frequently reported severe adverse reactions in elderly patients. In those concurrently receiving certain diuretics, primarily thiazides, an increased incidence of thrombocytopenia with purpura has been reported. Increased digoxin blood levels can occur with concomitant sulfamethoxazole and trimethoprim therapy, especially in elderly patients. Serum digoxin levels should be monitored. Hematological changes indicative of folic acid deficiency may occur in elderly patients. These effects are reversible by folinic acid therapy. Appropriate dosage adjustments should be made for patients with impaired kidney function and duration of use should be as short as possible to minimize risks of undesired reactions (see DOSAGE AND ADMINISTRATION). The trimethoprim component of sulfamethoxazole and trimethoprim may cause hyperkalemia when administered to patients with underlying disorders of potassium metabolism, with renal insufficiency or when given concomitantly with drugs known to induce hyperkalemia, such as angiotensin converting enzyme inhibitors. Close monitoring of serum potassium is warranted in these patients. Discontinuation of sulfamethoxazole and trimethoprim treatment is recommended to help lower potassium serum levels.
Pharmacokinetics parameters for sulfamethoxazole were similar for geriatric subjects and younger adult subjects. The mean maximum serum trimethoprim concentration was higher and mean renal clearance of trimethoprim was lower in geriatric subjects compared with younger subjects (see CLINICAL PHARMACOLOGY:Geriatric Pharmacokinetics).
Close -
ADVERSE REACTIONSThe following adverse reactions associated with the use of sulfamethoxazole and trimethoprim were identified in clinical trials, postmarketing or published reports. Because some of these reactions ...
The following adverse reactions associated with the use of sulfamethoxazole and trimethoprim were identified in clinical trials, postmarketing or published reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The most common adverse reactions are gastrointestinal disturbances (nausea, vomiting, anorexia) and allergic skin reactions (such as rash and urticaria). Fatalities and serious adverse reactions, including severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS), acute febrile neutrophilic dermatosis (AFND), acute generalized erythematous pustulosis (AGEP); fulminant hepatic necrosis; agranulocytosis, aplastic anemia and other blood dyscrasias; acute and delayed lung injury; anaphylaxis and circulatory shock have occurred with the administration of sulfamethoxazole and trimethoprim products, including sulfamethoxazole and trimethoprim (see WARNINGS).
Hematologic: Agranulocytosis, aplastic anemia, thrombocytopenia, leukopenia, neutropenia, hemolytic anemia, megaloblastic anemia, hypoprothrombinemia, methemoglobinemia, eosinophilia, thrombotic thrombocytopenic purpura, idiopathic thrombocytopenic purpura.
Allergic / Immune Reactions:Stevens-Johnson syndrome, toxic epidermal necrolysis, anaphylaxis, allergic myocarditis, erythema multiforme, exfoliative dermatitis, angioedema, drug fever, chills, Henoch-Schöenlein purpura, serum sickness-like syndrome, generalized allergic reactions, generalized skin eruptions, photosensitivity, conjunctival and scleral injection, pruritus, urticaria, rash, periarteritis nodosa, hemophagocytic lymphohistiocytosis (HLH), systemic lupus erythematosus, drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized erythematous pustulosis (AGEP), and acute febrile neutrophilic dermatosis (AFND) (see WARNINGS).
Gastrointestinal: Hepatitis (including cholestatic jaundice and hepatic necrosis), elevation of serum transaminase and bilirubin, pseudomembranous enterocolitis, pancreatitis, stomatitis, glossitis, nausea, emesis, abdominal pain, diarrhea, anorexia.
Genitourinary: Renal failure, interstitial nephritis, BUN and serum creatinine elevation, renal insufficiency, oliguria and anuria, crystalluria and nephrotoxicity in association with cyclosporine.
Metabolic and Nutritional: Hyperkalemia, hyponatremia (see PRECAUTIONS:Electrolyte Abnormalities), metabolic acidosis.
Neurologic: Aseptic meningitis, convulsions, peripheral neuritis, ataxia, vertigo, tinnitus, headache.
Psychiatric: Hallucinations, depression, apathy, nervousness.
Endocrine: The sulfonamides bear certain chemical similarities to some goitrogens, diuretics (acetazolamide and the thiazides) and oral hypoglycemic agents. Cross-sensitivity may exist with these agents. Diuresis and hypoglycemia have occurred.
Musculoskeletal: Arthralgia, myalgia, rhabdomyolysis.
Respiratory: Cough, shortness of breath and pulmonary infiltrates, acute eosinophilic pneumonia, acute and delayed lung injury, interstitial lung disease, acute respiratory failure (see WARNINGS).
Cardiovascular System:QT prolongation resulting in ventricular tachycardia and torsades de pointes, circulatory shock (see WARNINGS).
Miscellaneous: Weakness, fatigue, insomnia.
Close -
OVERDOSAGEAcute - The amount of a single dose of sulfamethoxazole and trimethoprim that is either associated with symptoms of overdosage or is likely to be life-threatening has not been reported. Signs and ...
Acute
The amount of a single dose of sulfamethoxazole and trimethoprim that is either associated with symptoms of overdosage or is likely to be life-threatening has not been reported. Signs and symptoms of overdosage reported with sulfonamides include anorexia, colic, nausea, vomiting, dizziness, headache, drowsiness and unconsciousness. Pyrexia, hematuria and crystalluria may be noted. Blood dyscrasias and jaundice are potential late manifestations of overdosage.
Signs of acute overdosage with trimethoprim include nausea, vomiting, dizziness, headache, mental depression, confusion and bone marrow depression.
General principles of treatment include the institution of gastric lavage or emesis, forcing oral fluids, and the administration of intravenous fluids if urine output is low and renal function is normal. Acidification of the urine will increase renal elimination of trimethoprim. The patient should be monitored with blood counts and appropriate blood chemistries, including electrolytes. If a significant blood dyscrasia or jaundice occurs, specific therapy should be instituted for these complications. Peritoneal dialysis is not effective and hemodialysis is only moderately effective in eliminating sulfamethoxazole and trimethoprim.
Chronic
Use of sulfamethoxazole and trimethoprim at high doses and/or for extended periods of time may cause bone marrow depression manifested as thrombocytopenia, leukopenia and/or megaloblastic anemia. If signs of bone marrow depression occur, the patient should be given leucovorin 5 to 15 mg daily until normal hematopoiesis is restored.
Close -
DOSAGE AND ADMINISTRATIONSulfamethoxazole and trimethoprim oral suspension is contraindicated in pediatric patients less than 2 months of age. Urinary Tract Infections and Shigellosis in Adults and Pediatric Patients ...
Sulfamethoxazole and trimethoprim oral suspension is contraindicated in pediatric patients less than 2 months of age.
Urinary Tract Infections and Shigellosis in Adults and Pediatric Patients, and Acute Otitis Media in Children
Adults: The usual adult dosage in the treatment of urinary tract infections is 4 teaspoonfuls (20 mL) of sulfamethoxazole and trimethoprim oral suspension every 12 hours for 10 to 14 days. An identical daily dosage is used for 5 days in the treatment of shigellosis.
Children: The recommended dose for children with urinary tract infections or acute otitis media is 40 mg/kg sulfamethoxazole and 8 mg/kg trimethoprim per 24 hours, given in two divided doses every 12 hours for 10 days. An identical daily dosage is used for 5 days in the treatment of shigellosis. The following table is a guideline for the attainment of this dosage:
Children 2 months of age or older:
Weight
Dose - every 12 hours
lb
kg
Teaspoonfuls
22
10
1 (5 mL)
44
20
2 (10 mL)
66
30
3 (15 mL)
88
40
4 (20 mL)
For Patients with Impaired Renal Function
When renal function is impaired, a reduced dosage should be employed using the following table:
Creatinine Clearance (mL/min)
Recommended Dosage Regimen
Above 30
Usual standard regimen
15-30
1⁄2 the usual regimen
Below 15
Use not recommended
Acute Exacerbations of Chronic Bronchitis in Adults
The usual adult dosage in the treatment of acute exacerbations of chronic bronchitis is 4 teaspoonfuls (20 mL) sulfamethoxazole and trimethoprim oral suspension every 12 hours for 14 days.
Pneumocystis jiroveciiPneumonia
Treatment
Adults and Children:
The recommended dosage for treatment of patients with documented Pneumocystis jiroveciipneumonia is 75 to 100 mg/kg sulfamethoxazole and 15 to 20 mg/kg trimethoprim per 24 hours given in equally divided doses every 6 hours for 14 to 21 days. 12The following table is a guideline for the upper limit of this dosage:
Weight
Dose - every 6 hours
lb
kg
Teaspoonfuls
18
8
1 (5 mL)
35
16
2 (10 mL)
53
24
3 (15 mL)
70
32
4 (20 mL)
88
40
5 (25 mL)
108
48
6 (30 mL)
141
64
8 (40 mL)
176
80
10 (50 mL)
For the lower limit dose (75 mg/kg sulfamethoxazole and 15 mg/kg trimethoprim per 24 hours) administer 75% of the dose in the above table.
Prophylaxis
Adults:
The recommended dosage for prophylaxis in adults is 4 teaspoonfuls (20 mL) of sulfamethoxazole and trimethoprim oral suspension daily. 13
Children:
For children, the recommended dose is 750 mg/m 2/day sulfamethoxazole with 150 mg/m 2/day trimethoprim given orally in equally divided doses twice a day, on 3 consecutive days per week. The total daily dose should not exceed 1,600 mg sulfamethoxazole and 320 mg trimethoprim. 14The following table is a guideline for the attainment of this dosage in children:
Body Surface Area
Dose - every 12 hours
(m 2)
Teaspoonfuls
0.26
1/2 (2.5 mL)
0.53
1 (5 mL)
1.06
2 (10 mL)
Traveler’s Diarrhea in Adults
For the treatment of traveler’s diarrhea, the usual adult dosage is 4 teaspoonfuls (20 mL) of sulfamethoxazole and trimethoprim oral suspension every 12 hours for 5 days.
Close -
HOW SUPPLIEDSulfamethoxazole and Trimethoprim Oral Suspension, USP (Cherry Flavor), opaque, pinkish orange suspension with a cherry aroma, containing 40 mg trimethoprim and 200 mg sulfamethoxazole in each ...
Sulfamethoxazole and Trimethoprim Oral Suspension, USP (Cherry Flavor), opaque, pinkish orange suspension with a cherry aroma, containing 40 mg trimethoprim and 200 mg sulfamethoxazole in each teaspoonful (5 mL), and is available in a one pint (473 mL) bottle NDC 62135-749-47, 20 mL Unit-Dose Cup NDC 62135-873-52 and 20 Unit-Dose Cups of 20 mL each NDC 62135-873-24.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature] and protect from light.
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
To report SUSPECTED ADVERSE REACTIONS, contact Chartwell RX, LLC. at 1-845-232-1683 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
REFERENCES
1. Kremers P, Duvivier J, Heusghem C. Pharmacokinetic Studies of Co-Trimoxazole in Man after Single and Repeated Doses. J Clin Pharmacol. Feb-Mar 1974;14:112–117.
2. Kaplan SA, et al. Pharmacokinetic Profile of Trimethoprim-Sulfamethoxazole in Man. J Infect Dis. Nov 1973; 128 (Suppl): S547–S555.
3. Varoquaux O, et al. Pharmacokinetics of the trimethoprim-sulfamethoxazole combination in the elderly. Br J Clin Pharmacol. 1985;20:575–581.
4. Safrin S, Lee BL, Sande MA. Adjunctive folinic acid with trimethoprim-sulfamethoxazole for Pneumocystis carinii pneumonia in AIDS patients is associated with an increased risk of therapeutic failure and death. J Infect Dis. 1994 Oct;170(4):912–7.
5. Marinella Mark A. 1999. Trimethoprim-induced hyperkalemia: An analysis of reported cases. Gerontol.45:209–212.
6. Margassery, S. and B. Bastani. 2002. Life threatening hyperkalemia and acidosis secondary to trimethoprim-sulfamethoxazole treatment. J. Nephrol.14:410–414.
7. Moh R, et al. Haematological changes in adults receiving a zidovudine-containing HAART regimen in combination with cotrimoxazole in Côte d'Ivoire. Antivir Ther. 2005;10(5):615-24.
8. Al-Khatib SM, LaPointe N, Kramer JM, Califf RM. What Clinicians Should Know About the QT Interval. JAMA. 2003;289(16):2120-2127.
9. Boyer EW, Stork C, Wang RY. Review: The Pharmacology and Toxicology of Dofetilide. Int J Med Toxicol. 2001;4(2):16.
10. Kosoglou T, Rocci ML Jr, Vlasses PH. Trimethoprim alters the disposition of procainamide and N-acetylprocainamide. Clin Pharmacol Ther. Oct 1988;44(4):467-77.
11. Brumfitt W, Pursell R. Trimethoprim/Sulfamethoxazole in the Treatment of Bacteriuria in Women. J Infect Dis. Nov 1973; 128 (Suppl): S657–S663.
12. Masur H. Prevention and treatment of Pneumocystis pneumonia. N Engl J Med.1992; 327: 1853–1880.
13. Recommendations for prophylaxis against Pneumocystis carinii pneumonia for adults and adolescents infected with human immunodeficiency virus. MMWR. 1992; 41(RR-4):1–11.
14. CDC Guidelines for prophylaxis against Pneumocystis carinii pneumonia for children infected with human immunodeficiency virus. MMWR. 1991; 40(RR-2):1–13.
All trademarks are the property of their respective owners.
Manufactured for:
Chartwell RX, LLC.
Congers, NY 10920
L71664
Rev. 03/2025
Close -
PACKAGE LABEL.PRINCIPAL DISPLAY PANELSulfamethoxazole and Trimethoprim Oral Suspension, USP 200 mg/40 mg per 5 mL - NDC 62135-749-47 - 473 mL Bottle Label - Sulfamethoxazole and Trimethoprim Oral Suspension, USP 800 mg/160 mg per ...
Sulfamethoxazole and Trimethoprim Oral Suspension, USP 200 mg/40 mg per 5 mL - NDC 62135-749-47 - 473 mL Bottle Label

Sulfamethoxazole and Trimethoprim Oral Suspension, USP 800 mg/160 mg per 20 mL - NDC 62135-873-52 - 20 mL Unit Dose Cup Label
Close
-
INGREDIENTS AND APPEARANCEProduct Information
SULFAMETHOXAZOLE AND TRIMETHOPRIM sulfamethoxazole and trimethoprim suspension Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62135-749 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFAMETHOXAZOLE (UNII: JE42381TNV) (SULFAMETHOXAZOLE - UNII:JE42381TNV) SULFAMETHOXAZOLE 200 mg in 5 mL TRIMETHOPRIM (UNII: AN164J8Y0X) (TRIMETHOPRIM - UNII:AN164J8Y0X) TRIMETHOPRIM 40 mg in 5 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM BENZOATE (UNII: OJ245FE5EU) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SORBITOL SOLUTION (UNII: 8KW3E207O2) Product Characteristics Color orange (opaque pinkish orange) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62135-749-47 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/11/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077785 01/24/2007 SULFAMETHOXAZOLE AND TRIMETHOPRIM sulfamethoxazole and trimethoprim suspension Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62135-873 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFAMETHOXAZOLE (UNII: JE42381TNV) (SULFAMETHOXAZOLE - UNII:JE42381TNV) SULFAMETHOXAZOLE 800 mg in 20 mL TRIMETHOPRIM (UNII: AN164J8Y0X) (TRIMETHOPRIM - UNII:AN164J8Y0X) TRIMETHOPRIM 160 mg in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM BENZOATE (UNII: OJ245FE5EU) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SORBITOL SOLUTION (UNII: 8KW3E207O2) Product Characteristics Color orange (opaque pinkish orange) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62135-873-24 2 in 1 BOX 05/30/2024 1 10 in 1 TRAY 1 NDC:62135-873-52 20 mL in 1 CUP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077785 01/24/2007
CloseLabeler - Chartwell RX, LLC (079394054)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
SULFAMETHOXAZOLE AND TRIMETHOPRIM suspension
Number of versions: 4
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Mar 31, 2025 | 5 (current) | download |
| Dec 26, 2024 | 4 | download |
| Jun 3, 2024 | 3 | download |
| Oct 17, 2023 | 1 | download |
RxNorm
SULFAMETHOXAZOLE AND TRIMETHOPRIM suspension
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 313134 | sulfamethoxazole 200 MG / trimethoprim 40 MG in 5 mL Oral Suspension | PSN |
| 2 | 313134 | sulfamethoxazole 40 MG/ML / trimethoprim 8 MG/ML Oral Suspension | SCD |
| 3 | 313134 | SMX 40 MG/ML / TMP 8 MG/ML Oral Suspension | SY |
| 4 | 313134 | sulfamethoxazole 100 MG / trimethoprim 20 MG per 2.5 ML Oral Suspension | SY |
| 5 | 313134 | sulfamethoxazole 200 MG / trimethoprim 40 MG per 5 ML Oral Suspension | SY |
| 6 | 313134 | sulfamethoxazole 800 MG / trimethoprim 160 MG per 20 ML Oral Suspension | SY |
Get Label RSS Feed for this Drug
SULFAMETHOXAZOLE AND TRIMETHOPRIM suspension
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=b5b7aba5-f93d-46bc-a3c2-712de1c5cff3
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
SULFAMETHOXAZOLE AND TRIMETHOPRIM suspension
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 62135-749-47 |
| 2 | 62135-873-24 |
| 3 | 62135-873-52 |


