Label: POLYMYXIN B- polymyxin b sulfate injection, powder, for solution
- NDC Code(s): 70594-049-01, 70594-049-02
- Packager: Xellia Pharmaceuticals USA LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 19, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx ONLY - To reduce the development of drug-resistant bacteria and maintain the effectiveness of polymyxin B and other antibacterial drugs, polymyxin B should be used only to treat or prevent ...
-
BOXED WARNING
(What is this?)
WARNING
CAUTION: WHEN THIS DRUG IS GIVEN INTRAMUSCULARLY AND/OR INTRATHECALLY, IT SHOULD BE GIVEN ONLY TO HOSPITALIZED PATIENTS, SO AS TO PROVIDE CONSTANT SUPERVISION BY A PHYSICIAN.
RENAL FUNCTION SHOULD BE CAREFULLY DETERMINED AND PATIENTS WITH RENAL DAMAGE AND NITROGEN RETENTION SHOULD HAVE REDUCED DOSAGE. PATIENTS WITH NEPHROTOXICITY DUE TO POLYMYXIN B SULFATE USUALLY SHOW ALBUMINURIA, CELLULAR CASTS, AND AZOTEMIA.
DIMINISHING URINE OUTPUT AND A RISING BUN ARE INDICATIONS FOR DISCONTINUING THERAPY WITH THIS DRUG.
NEUROTOXIC REACTIONS MAY BE MANIFESTED BY IRRITABILITY, WEAKNESS, DROWSINESS, ATAXIA, PERIORAL PARESTHESIA, NUMBNESS OF THE EXTREMITIES, AND BLURRING OF VISION. THESE ARE USUALLY ASSOCIATED WITH HIGH SERUM LEVELS FOUND IN PATIENTS WITH IMPAIRED RENAL FUNCTION AND/OR NEPHROTOXICITY.
THE CONCURRENT OR SEQUENTIAL USE OF OTHER NEUROTOXIC AND/OR NEPHROTOXIC DRUGS WITH POLYMYXIN B SULFATE, PARTICULARLY BACITRACIN, STREPTOMYCIN, NEOMYCIN, KANAMYCIN, GENTAMICIN, TOBRAMYCIN, AMIKACIN, CEPHALORIDINE, PAROMOMYCIN, VIOMYCIN, AND COLISTIN SHOULD BE AVOIDED.
THE NEUROTOXICITY OF POLYMYXIN B SULFATE CAN RESULT IN RESPIRATORY PARALYSIS FROM NEUROMUSCULAR BLOCKADE, ESPECIALLY WHEN THE DRUG IS GIVEN SOON AFTER ANESTHESIA AND/OR MUSCLE RELAXANTS.
USAGE IN PREGNANCY: THE SAFETY OF THIS DRUG IN HUMAN PREGNANCY HAS NOT BEEN ESTABLISHED.
Close -
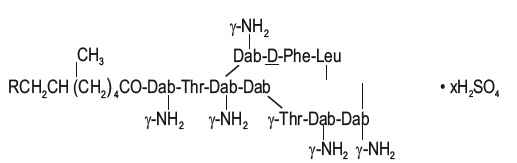
DESCRIPTIONPolymyxin B for Injection is one of a group of basic polypeptide antibiotics derived from B polymyxa (B aerosporous). Polymyxin B sulfate is the sulfate salt of Polymyxins B1 and B2, which are ...
-
CLINICAL PHARMACOLOGYPolymyxin B sulfate has a bactericidal action against almost all gram-negative bacilli except the Proteus group. Polymyxins increase the permeability of bacterial cell membrane leading to death of ...
-
INDICATIONS AND USAGEAcute Infections Caused by Susceptible Strains of Pseudomonas aeruginosa. Polymyxin B sulfate is a drug of choice in the treatment of infections of the urinary tract, meninges, and blood-stream ...
-
CONTRAINDICATIONSThis drug is contraindicated in persons with a prior history of hypersensitivity reactions to polymyxins.
-
WARNINGSClostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Polymyxin B for Injection, and may range in severity from mild diarrhea ...
-
PRECAUTIONSGeneral - Prescribing polymyxin B in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the ...
-
ADVERSE REACTIONSSee WARNING box. Nephrotoxic reactions - Albuminuria, cylinduria, azotemia, and rising blood levels without any increase in dosage. Neurotoxic reactions - Facial flushing, dizziness progressing ...
-
DOSAGE AND ADMINISTRATIONPARENTERAL - Intravenous - Dissolve 500,000 polymyxin B units in 300 to 500 mL solutions for parenteral 5% Dextrose Injection for continuous drip. Adults and children - 15,000 to 25,000 ...
-
HOW SUPPLIEDEach vial of Polymyxin B for Injection contains polymyxin B sulfate equivalent to 500,000 polymyxin B units. It is supplied in rubber-stoppered glass vial with flip off cap, as a single-vial ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Xellia Pharmaceuticals USA, LLC - Buffalo Grove, IL 60089 - Made in Hyderabad, Telangana, India - Revised: May 2024 - 1313000075-02 - Code No: AP/DRUGS/103/97
-
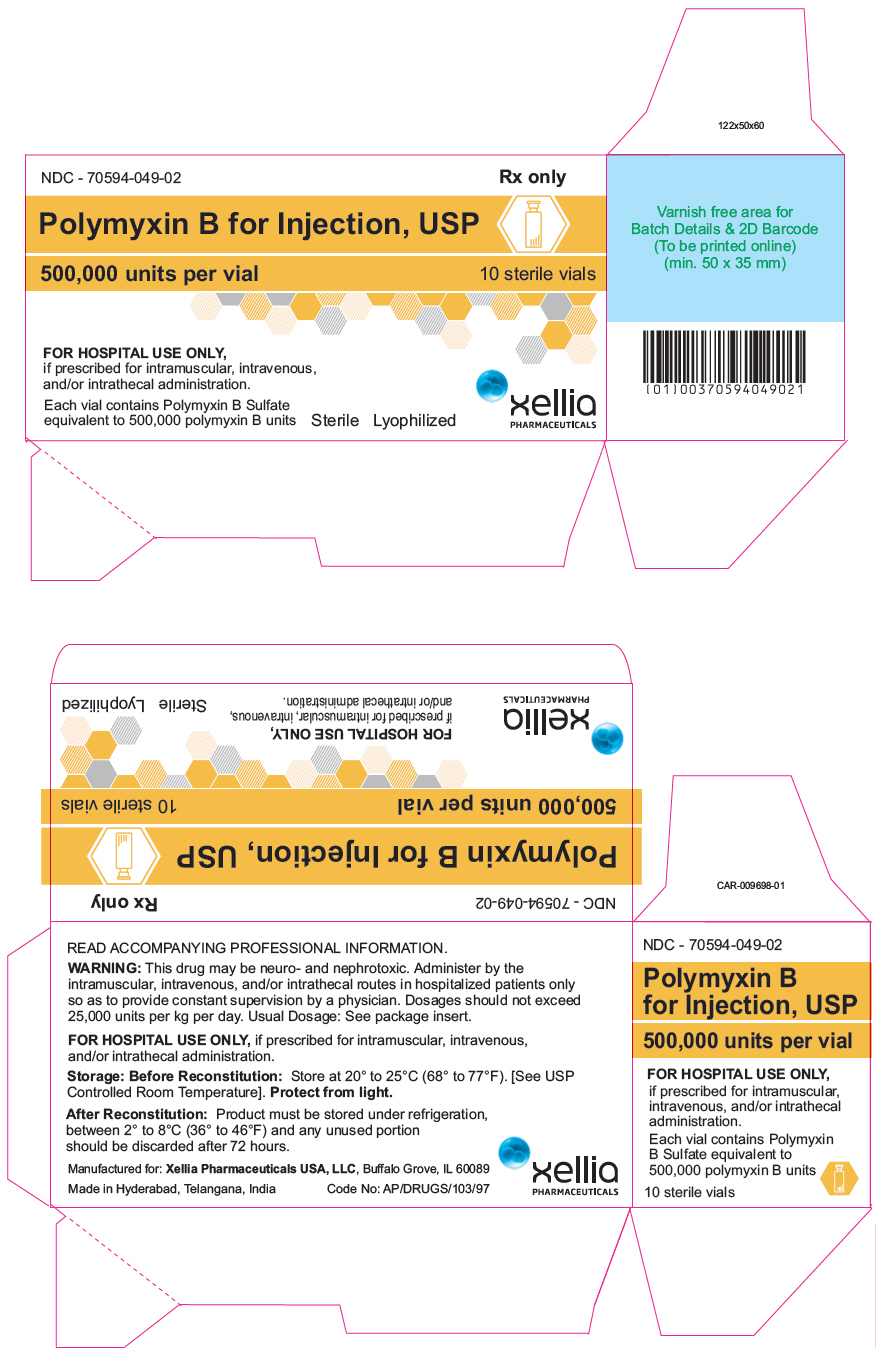
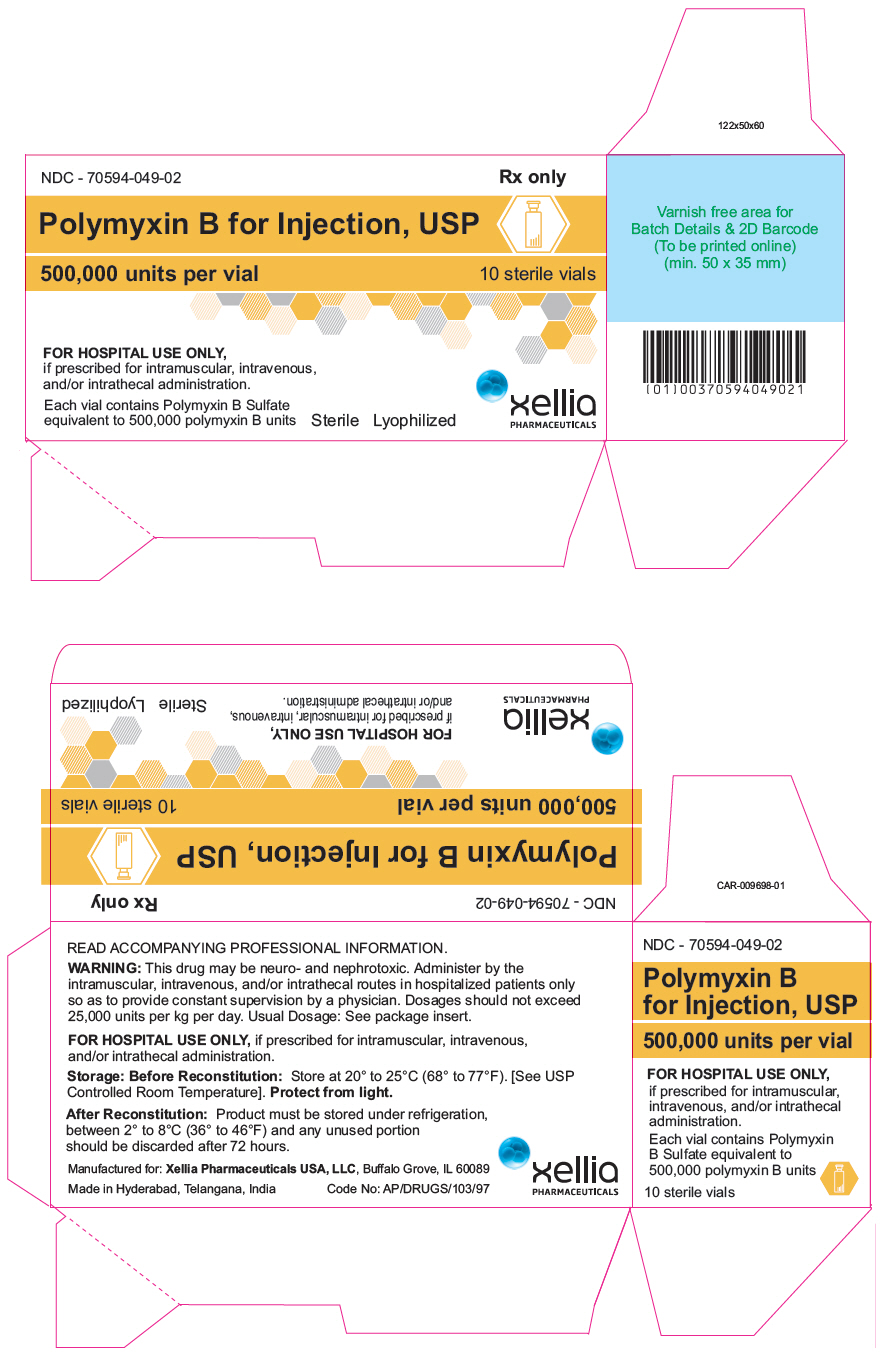
PRINCIPAL DISPLAY PANEL - 500,000 unit Vial CartonNDC - 70594-049-02 - Rx Only - Polymyxin B for Injection, USP - 500,000 units per vial - 10 sterile vials - FOR HOSPITAL USE ONLY, if prescribed for intramuscular, intravenous, and/or intrathecal ...
-
PRINCIPAL DISPLAY PANEL - 500,000 unit Vial LabelNDC 70594-049-01 - Polymyxin B - for Injection, USP - 500,000 units per vial - FOR HOSPITAL USE ONLY, if prescribed for intramuscular, intravenous, and/or intrathecal - administration. Rx Only - Sterile ...
-
PRINCIPAL DISPLAY PANEL - 500,000 unit Vial Carton - 1 VialNDC 70594-049-01 - Rx Only - Polymyxin B - for Injection, USP - 500,000 units per vial - FOR HOSPITAL USE ONLY, if prescribed for intramuscular, intravenous, and/or ...
-
INGREDIENTS AND APPEARANCEProduct Information