Label: DIAZEPAM tablet
-
NDC Code(s):
49999-018-02,
49999-018-05,
49999-018-10,
49999-018-15, view more49999-018-20, 49999-018-30, 49999-018-60, 49999-018-90
- Packager: Quality Care Products LLC
- This is a repackaged label.
- Source NDC Code(s): 0172-3926
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Diazepam is a benzodiazepine derivative. Chemically, diazepam is 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one. It is a colorless to light yellow crystalline compound, and is insoluble in water. Its structural formula is:
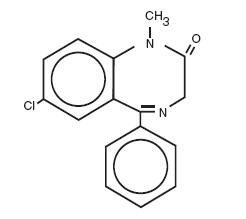
C16H13ClN2O M.W. 284.75
Diazepam is available as 2 mg, 5 mg, and 10 mg tablets for oral administration and contains the following inactive ingredients: anhydrous lactose, colloidal silicon dioxide; colorants: 5 mg only (D&C Yellow No. 10 and FD&C Yellow No. 6); 10 mg only (FD&C Blue No. 1); magnesium stearate, microcrystalline cellulose, pregelatinized starch, and sodium starch glycolate.
-
CLINICAL PHARMACOLOGY
Diazepam is a benzodiazepine that exerts anxiolytic, sedative, muscle-relaxant, anticonvulsant and amnestic effects. Most of these effects are thought to result from a facilitation of the action of gamma aminobutyric acid (GABA), an inhibitory neurotransmitter in the central nervous system.
Pharmacokinetics
Absorption
After oral administration > 90% of diazepam is absorbed and the average time to achieve peak plasma concentrations is 1 to 1.5 hours with a range of 0.25 to 2.5 hours. Absorption is delayed and decreased when administered with a moderate fat meal. In the presence of food mean lag times are approximately 45 minutes as compared with 15 minutes when fasting. There is also an increase in the average time to achieve peak concentrations to about 2.5 hours in the presence of food as compared with 1.25 hours when fasting. This results in an average decrease in Cmax of 20% in addition to a 27% decrease in AUC (range 15% to 50%) when administered with food.
Distribution
Diazepam and its metabolites are highly bound to plasma proteins (diazepam 98%). Diazepam and its metabolites cross the blood-brain and placental barriers and are also found in breast milk in concentrations approximately one tenth of those in maternal plasma (days 3 to 9 post-partum). In young healthy males, the volume of distribution at steady-state is 0.8 to 1 L/kg. The decline in the plasma concentration-time profile after oral administration is biphasic. The initial distribution phase has a half-life of approximately 1 hour, although it may range up to > 3 hours.
Metabolism
Diazepam is N-demethylated by CYP3A4 and 2C19 to the active metabolite N-desmethyldiazepam, and is hydroxylated by CYP3A4 to the active metabolite temazepam. N-desmethyldiazepam and temazepam are both further metabolized to oxazepam. Temazepam and oxazepam are largely eliminated by glucuronidation.
Elimination
The initial distribution phase is followed by a prolonged terminal elimination phase (half-life up to 48 hours). The terminal elimination half-life of the active metabolite N-desmethyldiazepam is up to 100 hours. Diazepam and its metabolites are excreted mainly in the urine, predominantly as their glucuronide conjugates. The clearance of diazepam is 20 to 30 mL/min in young adults. Diazepam accumulates upon multiple dosing and there is some evidence that the terminal elimination half-life is slightly prolonged.
Pharmacokinetics in Special Populations
Children
In children 3 to 8 years old the mean half-life of diazepam has been reported to be 18 hours.
Newborns
In full term infants, elimination half-lives around 30 hours have been reported, with a longer average half-life of 54 hours reported in premature infants of 28 to 34 weeks gestational age and 8 to 81 days post-partum. In both premature and full term infants the active metabolite desmethyldiazepam shows evidence of continued accumulation compared to children. Longer half-lives in infants may be due to incomplete maturation of metabolic pathways.
Geriatric
Elimination half-life increases by approximately 1 hour for each year of age beginning with a half-life of 20 hours at 20 years of age. This appears to be due to an increase in volume of distribution with age and a decrease in clearance. Consequently, the elderly may have lower peak concentrations, and on multiple dosing higher trough concentrations. It will also take longer to reach steady-state. Conflicting information has been published on changes of plasma protein binding in the elderly. Reported changes in free drug may be due to significant decreases in plasma proteins due to causes other than simply aging.
Hepatic Insufficiency
In mild and moderate cirrhosis, average half-life is increased. The average increase has been variously reported from 2 fold to 5 fold, with individual half-lives over 500 hours reported. There is also an increase in volume of distribution, and average clearance decreases by almost half. Mean half-life is also prolonged with hepatic fibrosis to 90 hours (range 66 to 104 hours), with chronic active hepatitis to 60 hours (range 26 to 76 hours), and with acute viral hepatitis to 74 hours (range 49 to 129). In chronic active hepatitis, clearance is decreased by almost half.
-
INDICATIONS AND USAGE
Diazepam Tablets USP are indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
In acute alcohol withdrawal, diazepam may be useful in the symptomatic relief of acute agitation, tremor, impending or acute delirium tremens and hallucinosis.
Diazepam is a useful adjunct for the relief of skeletal muscle spasm due to reflex spasm to local pathology (such as inflammation of the muscles or joints, or secondary to trauma), spasticity caused by upper motor neuron disorders (such as cerebral palsy and paraplegia), athetosis, and stiff-man syndrome.
Oral diazepam may be used adjunctively in convulsive disorders, although it has not proved useful as the sole therapy.
The effectiveness of diazepam in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
-
CONTRAINDICATIONS
Diazepam Tablets USP are contraindicated in patients with a known hypersensitivity to diazepam and, because of lack of sufficient clinical experience, in pediatric patients under 6 months of age. Diazepam Tablets USP are also contraindicated in patients with myasthenia gravis, severe respiratory insufficiency, severe hepatic insufficiency, and sleep apnea syndrome. They may be used in patients with open-angle glaucoma who are receiving appropriate therapy, but are contraindicated in acute narrow-angle glaucoma.
-
WARNINGS
Diazepam is not recommended in the treatment of psychotic patients and should not be employed instead of appropriate treatment.
Since diazepam has a central nervous system depressant effect, patients should be advised against the simultaneous ingestion of alcohol and other CNS-depressant drugs during diazepam therapy.
As with other agents that have anticonvulsant activity, when diazepam is used as an adjunct in treating convulsive disorders, the possibility of an increase in the frequency and/or severity of grand mal seizures may require an increase in the dosage of standard anticonvulsant medication. Abrupt withdrawal of diazepam in such cases may also be associated with a temporary increase in the frequency and/or severity of seizures.
Pregnancy
An increased risk of congenital malformations and other developmental abnormalities associated with the use of benzodiazepine drugs during pregnancy has been suggested. There may also be non-teratogenic risks associated with the use of benzodiazepines during pregnancy. There have been reports of neonatal flaccidity, respiratory and feeding difficulties, and hypothermia in children born to mothers who have been receiving benzodiazepines late in pregnancy. In addition, children born to mothers receiving benzodiazepines on a regular basis late in pregnancy may be at some risk of experiencing withdrawal symptoms during the postnatal period.
Diazepam has been shown to be teratogenic in mice and hamsters when given orally at daily doses of 100 mg/kg or greater (approximately eight times the maximum recommended human dose [MRHD = 1 mg/kg/day] or greater on a mg/m2 basis). Cleft palate and encephalopathy are the most common and consistently reported malformations produced in these species by administration of high, maternally toxic doses of diazepam during organogenesis. Rodent studies have indicated that prenatal exposure to diazepam doses similar to those used clinically can produce long-term changes in cellular immune responses, brain neurochemistry, and behavior.
In general, the use of diazepam in women of childbearing potential, and more specifically during known pregnancy, should be considered only when the clinical situation warrants the risk to the fetus. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Patients should also be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physician about the desirability of discontinuing the drug.
Labor and Delivery
Special care must be taken when diazepam is used during labor and delivery, as high single doses may produce irregularities in the fetal heart rate and hypotonia, poor sucking, hypothermia, and moderate respiratory depression in the neonates. With newborn infants it must be remembered that the enzyme system involved in the breakdown of the drug is not yet fully developed (especially in premature infants).
-
PRECAUTIONS
General
If diazepam is to be combined with other psychotropic agents or anticonvulsant drugs, careful consideration should be given to the pharmacology of the agents to be employed - particularly with known compounds that may potentiate the action of diazepam, such as phenothiazines, narcotics, barbiturates, MAO inhibitors and other antidepressants (see Drug Interactions).
The usual precautions are indicated for severely depressed patients or those in whom there is any evidence of latent depression or anxiety associated with depression, particularly the recognition that suicidal tendencies may be present and protective measures may be necessary.
Psychiatric and paradoxical reactions are known to occur when using benzodiazepines (see ADVERSE REACTIONS). Should this occur, use of the drug should be discontinued. These reactions are more likely to occur in children and the elderly.
A lower dose is recommended for patients with chronic respiratory insufficiency, due to the risk of respiratory depression.
Benzodiazepines should be used with extreme caution in patients with a history of alcohol or drug abuse (see DRUG ABUSE AND DEPENDENCE).
In debilitated patients, it is recommended that the dosage be limited to the smallest effective amount to preclude the development of ataxia or oversedation (2 mg to 2.5 mg once or twice daily, initially, to be increased gradually as needed and tolerated).
Some loss of response to the effects of benzodiazepines may develop after repeated use of diazepam for a prolonged time.
Information for Patients
To assure the safe and effective use of benzodiazepines, patients should be informed that, since benzodiazepines may produce psychological and physical dependence, it is advisable that they consult with their physician before either increasing the dose or abruptly discontinuing this drug. The risk of dependence increases with duration of treatment; it is also greater in patients with a history of alcohol or drug abuse.
Patients should be advised against the simultaneous ingestion of alcohol and other CNS-depressant drugs during diazepam therapy. As is true of most CNS-acting drugs, patients receiving diazepam should be cautioned against engaging in hazardous occupations requiring complete mental alertness, such as operating machinery or driving a motor vehicle.
Drug Interactions
Centrally Acting Agents
If diazepam is to be combined with other centrally acting agents, careful consideration should be given to the pharmacology of the agents employed particularly with compounds that may potentiate or be potentiated by the action of diazepam, such as phenothiazines, antipsychotics, anxiolytics/sedatives, hypnotics, anticonvulsants, narcotic analgesics, anesthetics, sedative antihistamines, narcotics, barbiturates, MAO inhibitors and other antidepressants.
Antacids
Diazepam peak concentrations are 30% lower when antacids are administered concurrently. However, there is no effect on the extent of absorption. The lower peak concentrations appear due to a slower rate of absorption, with the time required to achieve peak concentrations on average 20 to 25 minutes greater in the presence of antacids. However, this difference was not statistically significant.
Compounds Which Inhibit Certain Hepatic Enzymes
There is a potentially relevant interaction between diazepam and compounds which inhibit certain hepatic enzymes (particularly cytochrome P450 3A and 2C19). Data indicate that these compounds influence the pharmacokinetics of diazepam and may lead to increased and prolonged sedation. At present, this reaction is known to occur with cimetidine, ketoconazole, fluvoxamine, fluoxetine, and omeprazole.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In studies in which mice and rats were administered diazepam in the diet at a dose of 75 mg/kg/day (approximately 6 and 12 times, respectively, the maximum recommended human dose [MRHD = 1 mg/kg/day] on a mg/m2 basis) for 80 and 104 weeks, respectively, an increased incidence of liver tumors was observed in males of both species. The data currently available are inadequate to determine the mutagenic potential of diazepam. Reproduction studies in rats showed decreases in the number of pregnancies and in the number of surviving offspring following administration of an oral dose of 100 mg/kg/day (approximately 16 times the MRHD on a mg/m2 basis) prior to and during mating and throughout gestation and lactation. No adverse effects on fertility or offspring viability were noted at a dose of 80 mg/kg/day (approximately 13 times the MRHD on a mg/m2 basis).
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 6 months have not been established.
Geriatric Use
In elderly patients, it is recommended that the dosage be limited to the smallest effective amount to preclude the development of ataxia or oversedation (2 mg to 2.5 mg once or twice daily, initially to be increased gradually as needed and tolerated).
Extensive accumulation of diazepam and its major metabolite, desmethyldiazepam, has been noted following chronic administration of diazepam in healthy elderly male subjects. Metabolites of this drug are known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Hepatic Insufficiency
Decreases in clearance and protein binding, and increases in volume of distribution and half-life has been reported in patients with cirrhosis. In such patients, a 2 to 5 fold increase in mean half-life has been reported. Delayed elimination has also been reported for the active metabolite desmethyldiazepam. Benzodiazepines are commonly implicated in hepatic encephalopathy. Increases in half-life have also been reported in hepatic fibrosis and in both acute and chronic hepatitis (see CLINICAL PHARMACOLOGY, Pharmacokinetics in Special Populations,Hepatic Insufficiency).
-
ADVERSE REACTIONS
Side effects most commonly reported were drowsiness, fatigue, muscle weakness, and ataxia. The following have also been reported:
Central Nervous System: confusion, depression, dysarthria, headache, slurred speech, tremor, vertigo
Gastrointestinal System: constipation, nausea, gastrointestinal disturbances
Special Senses: blurred vision, diplopia, dizziness
Cardiovascular System: hypotension
Psychiatric and Paradoxical Reactions: stimulation, restlessness, acute hyperexcited states, anxiety, agitation, aggressiveness, irritability, rage, hallucinations, psychoses, delusions, increased muscle spasticity, insomnia, sleep disturbances, and nightmares. Inappropriate behavior and other adverse behavioral effects have been reported when using benzodiazepines. Should these occur, use of the drug should be discontinued. They are more likely to occur in children and in the elderly.
Urogenital System: incontinence, changes in libido, urinary retention
Skin and Appendages: skin reactions
Laboratories: elevated transaminases and alkaline phosphatase
Other: changes in salivation, including dry mouth, hypersalivation
Antegrade amnesia may occur using therapeutic dosages, the risk increasing at higher dosages. Amnestic effects may be associated with inappropriate behavior.
Minor changes in EEG patterns, usually low-voltage fast activity, have been observed in patients during and after diazepam therapy and are of no known significance.
Because of isolated reports of neutropenia and jaundice, periodic blood counts and liver function tests are advisable during long-term therapy.
-
DRUG ABUSE AND DEPENDENCE
Diazepam is subject to Schedule IV control under the Controlled Substances Act of 1970. Abuse and dependence of benzodiazepines have been reported. Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving diazepam or other psychotropic agents because of the predisposition of such patients to habituation and dependence. Once physical dependence to benzodiazepines has developed, termination of treatment will be accompanied by withdrawal symptoms. The risk is more pronounced in patients on long-term therapy.
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol have occurred following abrupt discontinuance of diazepam. These withdrawal symptoms may consist of tremor, abdominal and muscle cramps, vomiting, sweating, headache, muscle pain, extreme anxiety, tension, restlessness, confusion and irritability. In severe cases, the following symptoms may occur: derealization, depersonalization, hyperacusis, numbness and tingling of the extremities, hypersensitivity to light, noise and physical contact, hallucinations or epileptic seizures. The more severe withdrawal symptoms have usually been limited to those patients who had received excessive doses over an extended period of time. Generally milder withdrawal symptoms (e.g., dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy, abrupt discontinuation should generally be avoided and a gradual dosage tapering schedule followed.
Chronic use (even at therapeutic doses) may lead to the development of physical dependence: discontinuation of the therapy may result in withdrawal or rebound phenomena.
Rebound Anxiety: A transient syndrome whereby the symptoms that led to treatment with diazepam recur in an enhanced form. This may occur upon discontinuation of treatment. It may be accompanied by other reactions including mood changes, anxiety, and restlessness.
Since the risk of withdrawal phenomena and rebound phenomena is greater after abrupt discontinuation of treatment, it is recommended that the dosage be decreased gradually.
-
OVERDOSAGE
Overdose of benzodiazepines is usually manifested by central nervous system depression ranging from drowsiness to coma. In mild cases, symptoms include drowsiness, confusion, and lethargy. In more serious cases, symptoms may include ataxia, diminished reflexes, hypotonia, hypotension, respiratory depression, coma (rarely), and death (very rarely). Overdose of benzodiazepines in combination with other CNS depressants (including alcohol) may be fatal and should be closely monitored.
Management of Overdosage
Following overdose with oral benzodiazepines, general supportive measures should be employed including the monitoring of respiration, pulse, and blood pressure. Vomiting should be induced (within 1 hour) if the patient is conscious. Gastric lavage should be undertaken with the airway protected if the patient is unconscious. Intravenous fluids should be administered. If there is no advantage in emptying the stomach, activated charcoal should be given to reduce absorption. Special attention should be paid to respiratory and cardiac function in intensive care. General supportive measures should be employed, along with intravenous fluids, and an adequate airway maintained.
Should hypotension develop, treatment may include intravenous fluid therapy, repositioning, judicious use of vasopressors appropriate to the clinical situation, if indicated, and other appropriate countermeasures. Dialysis is of limited value.
As with the management of intentional overdosage with any drug, it should be considered that multiple agents may have been ingested.
Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for resedation, respiratory depression and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. Caution should be observed in the use of flumazenil in epileptic patients treated with benzodiazepines. The complete flumazenil package insert, including CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS, should be consulted prior to use.
Withdrawal symptoms of the barbiturate type have occurred after the discontinuation of benzodiazepines (see DRUG ABUSE AND DEPENDENCE).
-
DOSAGE AND ADMINISTRATION
Dosage should be individualized for maximum beneficial effect. While the usual daily dosages given below will meet the needs of most patients, there will be some who may require higher doses. In such cases dosage should be increased cautiously to avoid adverse effects.
ADULTS: USUAL DAILY DOSE Management of Anxiety Disorders and Relief of Symptoms of Anxiety Depending upon severity of symptoms – 2 mg to 10 mg, 2 to 4 times daily Symptomatic Relief in Acute Alcohol Withdrawal 10 mg, 3 or 4 times during the first 24 hours, reducing to 5 mg, 3 or 4 times daily as needed Adjunctively for Relief of Skeletal Muscle Spasm 2 mg to 10 mg, 3 or 4 times daily Adjunctively in Convulsive Disorders 2 mg to 10 mg, 2 to 4 times daily Geriatric Patients, or in the presence of debilitating disease 2 mg to 2.5 mg, 1 or 2 times daily initially; increase gradually as needed and tolerated PEDIATRIC PATIENTS: Because of varied responses to CNS-acting drugs, initiate therapy with lowest dose and increase as required. Not for use in pediatric patients under 6 months. 1 mg to 2.5 mg, 3 or 4 times daily initially; increase gradually as needed and tolerated -
HOW SUPPLIED
Diazepam Tablets USP, 2 mg are available as white, round, flat face, beveled edge tablets, debossed “3925” and bisected on one side and “TEVA” on the other side, containing 2 mg of diazepam USP
Diazepam Tablets USP, 5 mg are available as yellow, round, flat face, beveled edge tablets, debossed “3926” and bisected on one side and “TEVA” on the other side, containing 5 mg of diazepam USP
49999-018-10
49999-018-20
49999-018-30
49999-018-60
49999-018-90
Diazepam Tablets USP, 10 mg are available as light blue, round, flat face, beveled edge tablets,
debossed “3927” and bisected on one side and “TEVA” on the other side, containing 10 mg of diazepam USP
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Manufactured In Czech Republic By:
TEVA CZECH INDUSTRIES s.r.o.
Opava-Komarov, Czech Republic
Manufactured For:
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Rev. A 3/2010
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIAZEPAM
diazepam tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49999-018(NDC:0172-3926) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Diazepam (UNII: Q3JTX2Q7TU) (Diazepam - UNII:Q3JTX2Q7TU) Diazepam 5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color yellow Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code 3926;TEVA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49999-018-02 2 in 1 BOTTLE; Type 0: Not a Combination Product 11/29/2011 06/01/2014 2 NDC:49999-018-05 5 in 1 BOTTLE; Type 0: Not a Combination Product 11/29/2011 06/01/2014 3 NDC:49999-018-10 10 in 1 BOTTLE; Type 0: Not a Combination Product 11/29/2011 10/31/2025 4 NDC:49999-018-15 15 in 1 BOTTLE; Type 0: Not a Combination Product 11/29/2011 06/01/2014 5 NDC:49999-018-20 20 in 1 BOTTLE; Type 0: Not a Combination Product 11/29/2011 01/31/2021 6 NDC:49999-018-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 11/29/2011 01/31/2026 7 NDC:49999-018-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 11/29/2011 10/31/2025 8 NDC:49999-018-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 11/29/2011 08/31/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA071321 11/29/2011 01/31/2026 Labeler - Quality Care Products LLC (831276758) Establishment Name Address ID/FEI Business Operations Quality Care Products LLC 831276758 repack(49999-018)


