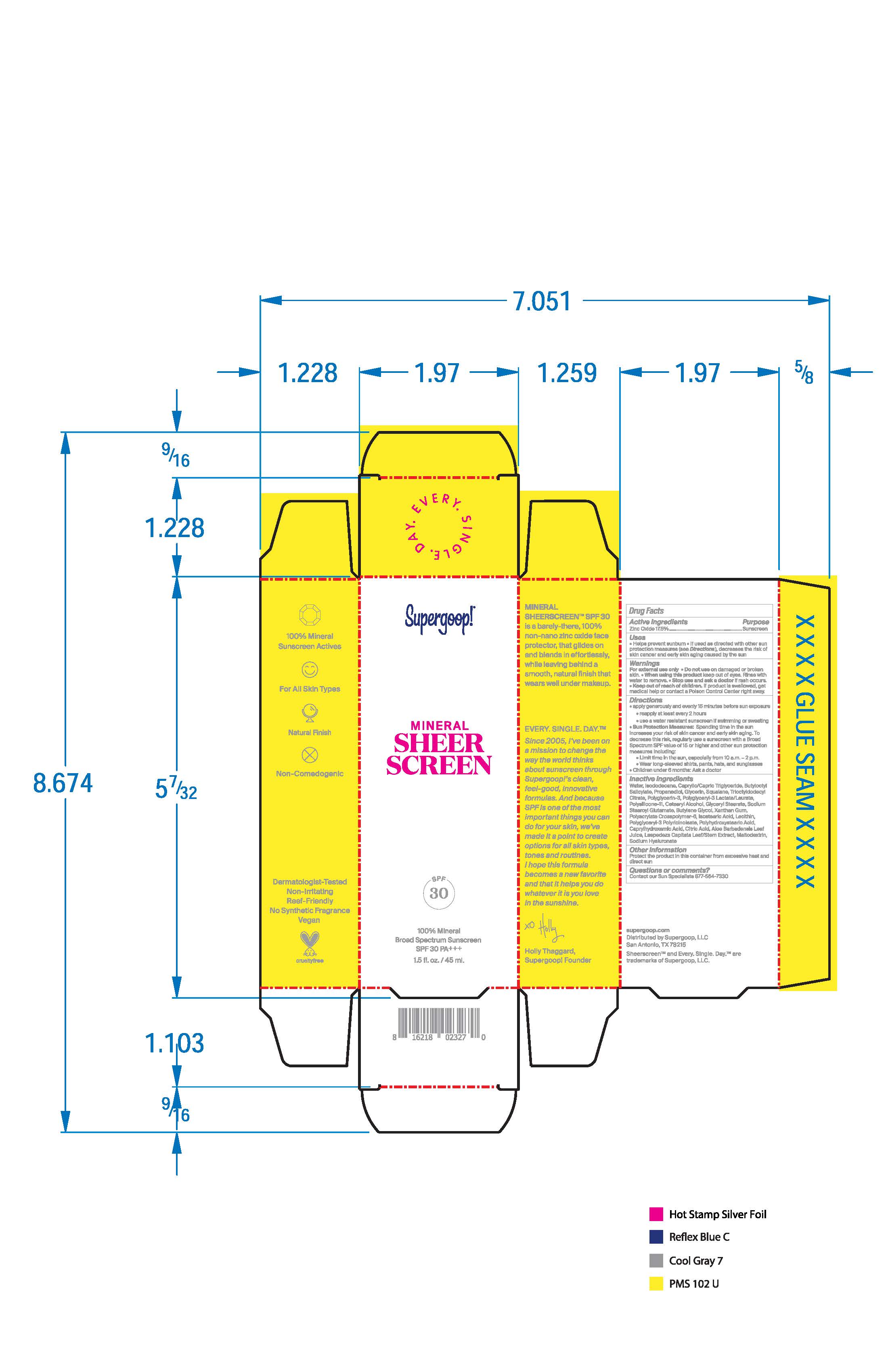
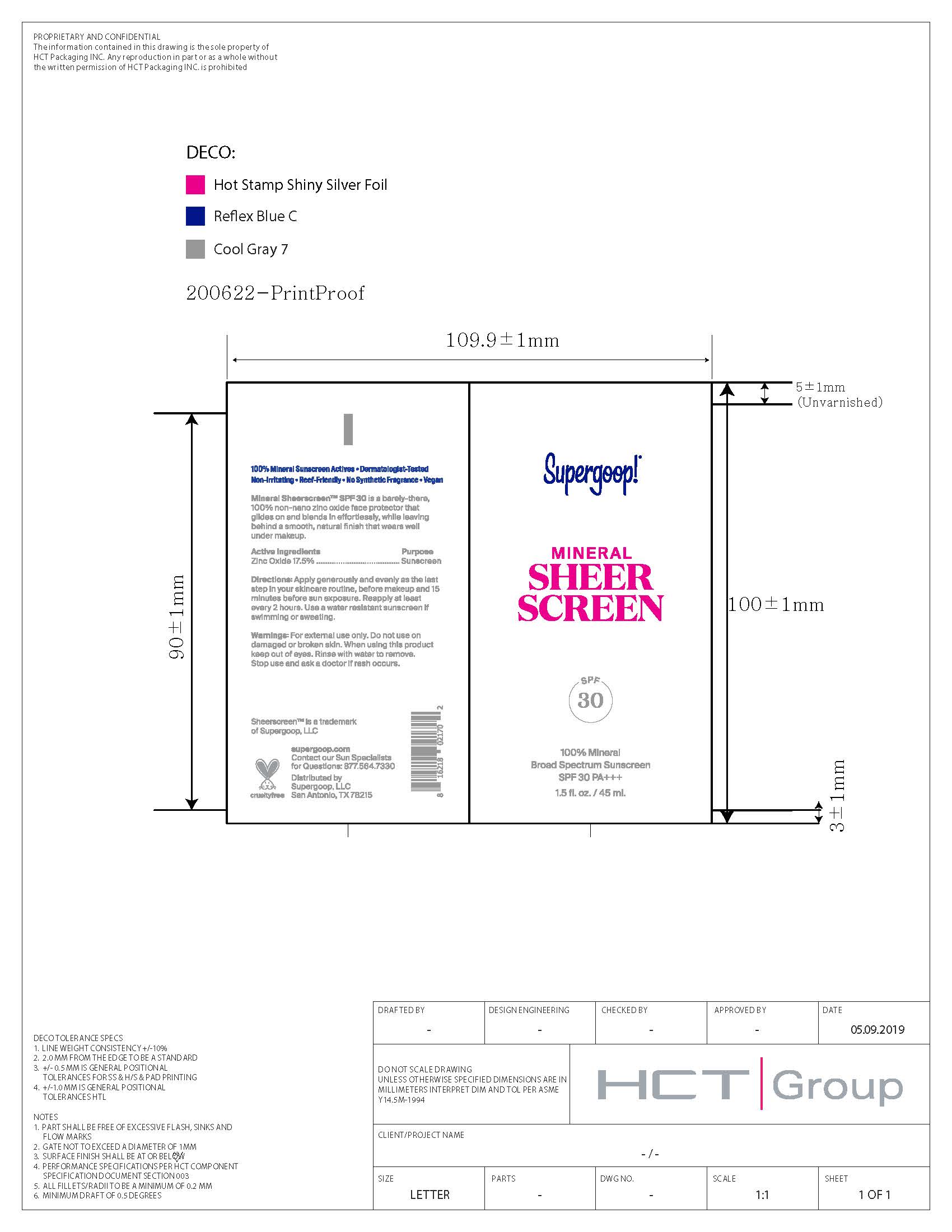
Label: MINERAL SHEER SCREEN BROAD SPECTRUM SUNSCREEN SPF 30- zinc oxide lotion
-
NDC Code(s):
75936-305-01,
75936-305-02,
75936-305-03,
75936-305-04, view more75936-305-05
- Packager: Supergoop LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
- apply generously and evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglassess
- Children under 6 months: Ask a doctor.
- apply generously and evenly 15 minutes before sun exposure
-
INACTIVE INGREDIENT
Water, Isododecane, Caprylic/Capric Triglyceride, Butyloctyl Salicylate, Propanedlol, Glycerin, Squalane, Trioctyldodecyl Citrate, Polyglycerln-3, Polyglyceryl-3 Lactate/Laurate, Polysilicone-11, Cetearyl Alcohol, Glyceryl Stearate, Sodium Stearoyl Glutamate, Butylene Glycol, Xanthan Gum, Polyacrylate Crosspolymer-6, lsostearic Acid, Lecithin, Polyglyceryl-3 Polyricinoleate, Polyhydroxystearic Acid, Caprylhydroxamic Acid, Citric Acid, Aloe Barbadensis Leaf Juice, Lespedeza Capitata Leaf/Stem Extract, Maltodextrin, Sodium Hyaluronate
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MINERAL SHEER SCREEN BROAD SPECTRUM SUNSCREEN SPF 30
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-305 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 17.5 g in 100 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERIN (UNII: PDC6A3C0OX) TRIOCTYLDODECYL CITRATE (UNII: 35X8CT063R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) ALOE VERA LEAF (UNII: ZY81Z83H0X) HYALURONATE SODIUM (UNII: YSE9PPT4TH) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PROPANEDIOL (UNII: 5965N8W85T) SQUALANE (UNII: GW89575KF9) ISOSTEARIC ACID (UNII: X33R8U0062) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) XANTHAN GUM (UNII: TTV12P4NEE) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) MALTODEXTRIN (UNII: 7CVR7L4A2D) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-305-01 45 mL in 1 TUBE; Type 0: Not a Combination Product 01/12/2021 2 NDC:75936-305-02 15 mL in 1 TUBE; Type 0: Not a Combination Product 01/12/2021 3 NDC:75936-305-03 10 mL in 1 TUBE; Type 0: Not a Combination Product 01/12/2021 4 NDC:75936-305-04 5 mL in 1 TUBE; Type 0: Not a Combination Product 01/12/2021 5 NDC:75936-305-05 20 mL in 1 TUBE; Type 0: Not a Combination Product 08/26/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/12/2021 Labeler - Supergoop LLC (117061743)