Label: TOPICAL ANALGESIC- menthol cream
- NDC Code(s): 80986-101-01
- Packager: Youngevity International Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 26, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active IngredientMenthol 1.4%, Purpose: Topical Analgesic
-
PurposeTopical Analgesic
-
UsesTemporary relief on minor aches and pains of sore muscles and joints associated with: Backaches - arthritis - strains - bruises - sprains
-
WarningsFor external use only.
-
Stop use and ask a doctorStop use and ask a doctor if - condition worsens - symptoms persists for more than 7 day or clears up and occurs again within a few days
-
When using this productWhen using this product: Keep out of eyes, ears, and mouth - In case of contact with eyes, rinse eyes thoroughly with water - Do not apply to wounds or damaged skin - Do not bandage tightly
-
Keep out of reach of childrenIf swallowed, get medical help or contact a Poison Control Center right away.
-
Directionsadults and children 2 years of age or older, apply to affected area no more than 3 to 4 times a day. children under 2 years of age, consult a doctor.
-
Other informationStore in a cool, dry place
-
Inactive IngredientCarbomer, celadrin (cetyl myristoleate, cetyl myristate, cetyl palmitaoleate, cetyl oleate, cetyl laurate), cetyl alcohol, ethylhexylglycerin, glycerin, glyceryl monostearate, myristyl alcohol ...
-
PDPUltimate CM Cream Muscle and Joint Support 2 fl oz
-
INGREDIENTS AND APPEARANCEProduct Information

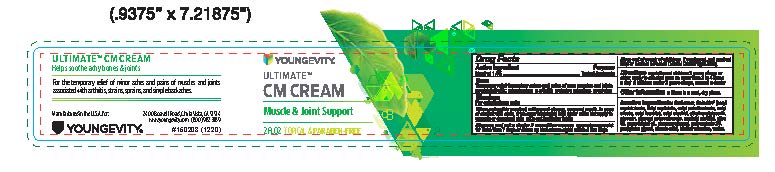
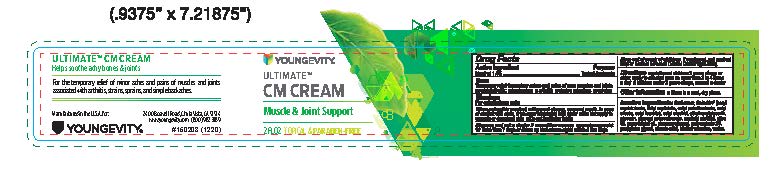 Ultimate CM Cream Muscle and Joint Support 2 fl oz
Ultimate CM Cream Muscle and Joint Support 2 fl oz