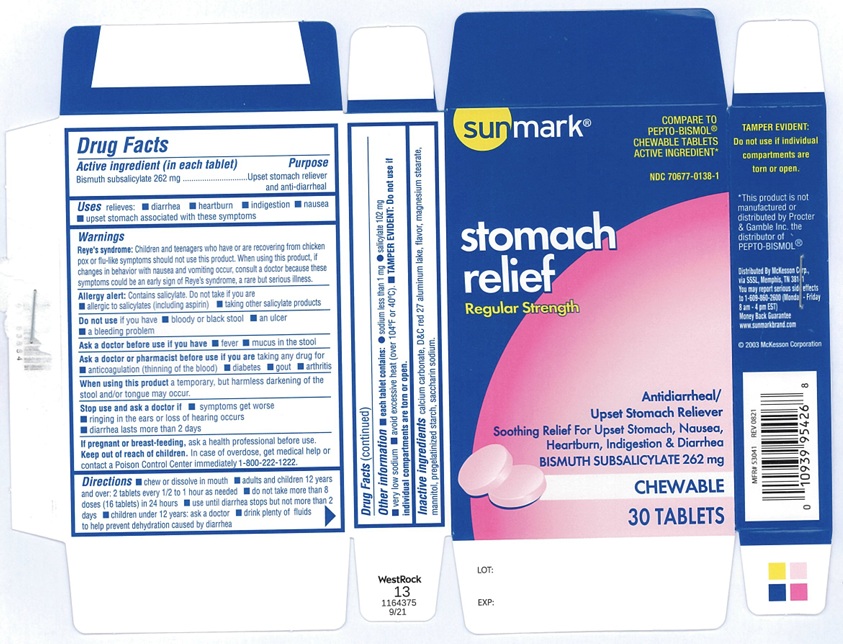
Label: SUNMARK STOMACH RELIEF- bismuth subsalicylate tablet, chewable
- NDC Code(s): 70677-0138-1
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT(in each tablet)
- PURPOSE
- USE(S)
-
WARNINGS
Reye's Syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behaviour with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's Syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
- allergic to salicylates (including aspirin)
- taking other salicylate products
- DO NOT USE IF YOU HAVE
- ASK A DOCTOR BEFORE USE IF YOU HAVE
- ASK A DOCTOR OR PHARMACIST BEFORE USE IF YOU ARE
- WHEN USING THIS PRODUCT
- STOP USE AND ASK DOCTOR IF
- IF PREGNANT OR BREAST-FEEDING,
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
- chew or dissolve in mouth
- adults and children 12 years and over: 2 tablets every 1/2 to 1 hour as needed
- do not take more than 8 doses (16 tablets) in 24 hours
- use until diarrhea stops but not more than 2 days
- children under 12 years: ask a doctor
- drink plenty of fluids to help prevent dehydration caused diarrhea
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUNMARK STOMACH RELIEF
bismuth subsalicylate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70677-0138 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISMUTH SUBSALICYLATE (UNII: 62TEY51RR1) (SALICYLIC ACID - UNII:O414PZ4LPZ) BISMUTH SUBSALICYLATE 262 mg Inactive Ingredients Ingredient Name Strength CALCIUM CARBONATE (UNII: H0G9379FGK) D&C RED NO. 27 (UNII: 2LRS185U6K) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color PINK Score no score Shape ROUND Size 16mm Flavor Imprint Code G122 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70677-0138-1 5 in 1 BOX 09/08/2021 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M008 09/08/2021 12/13/2024 Labeler - Strategic Sourcing Services LLC (116956644) Establishment Name Address ID/FEI Business Operations Guardian Drug Company 119210276 MANUFACTURE(70677-0138)