Label: DOXYCYCLINE HYCLATE tablet, film coated
- NDC Code(s): 62135-623-90
- Packager: Chartwell RX, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 15, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of Doxycycline Hyclate Tablets, USP and other antibacterial drugs, Doxycycline Hyclate Tablets, USP should be ...
-
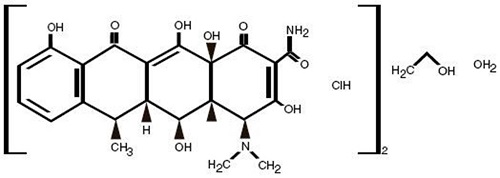
DESCRIPTIONDoxycycline Hyclate Tablets, USP are available as a 20 mg formulation of doxycycline for oral administration. The structural formula of doxycycline hyclate is: with an empirical formula of (C ...
-
CLINICAL PHARMACOLOGYAfter oral administration, doxycycline hyclate is rapidly and nearly completely absorbed from the gastrointestinal tract. Doxycycline is eliminated with a half-life of approximately 18 hours by ...
-
INDICATIONS AND USAGEDoxycycline hyclate tablets are indicated for use as an adjunct to scaling and root planing to promote attachment level gain and to reduce pocket depth in patients with adult periodontitis. To ...
-
CONTRAINDICATIONSThis drug is contraindicated in persons who have shown hypersensitivity to doxycycline or any of the other tetracyclines.
-
WARNINGSTHE USE OF DRUGS OF THE TETRACYCLINE CLASS DURING TOOTH DEVELOPMENT (LAST HALF OF PREGNANCY, INFANCY AND CHILDHOOD TO THE AGE OF 8 YEARS) MAY CAUSE PERMANENT DIS-COLORATION OF THE TEETH ...
-
PRECAUTIONSPrescribing Doxycycline Hyclate Tablets, USP in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and ...
-
ADVERSE REACTIONSAdverse Reactions in Clinical Trials of a bioequivalent form of doxycycline hyclate capsules:In clinical trials of adult patients with periodontal disease 213 patients received 20 mg BID over a ...
-
OVERDOSAGEIn case of overdosage, discontinue medication, treat symptomatically and institute supportive measures. Dialysis does not alter serum half-life and thus would not be of benefit in treating cases ...
-
DOSAGE AND ADMINISTRATIONTHE DOSAGE OF DOXYCYCLINE HYCLATE TABLETS, USP DIFFERS FROM THAT OF DOXYCYCLINE USED TO TREAT INFECTIONS. EXCEEDING THE RECOMMENDED DOSAGE MAY RESULT IN AN INCREASED INCIDENCE OF SIDE EFFECTS ...
-
HOW SUPPLIEDDoxycycline Hyclate Tablets, USP (equivalent to 20 mg doxycycline) are round, off-white, film coated tablets, debossed with “ CE” on one side and “ 115” on the other side. The tablets ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANELDoxycycline Hyclate Tablets, USP 20mg NDC 62135-623-90 90's Bottle Label
-
INGREDIENTS AND APPEARANCEProduct Information