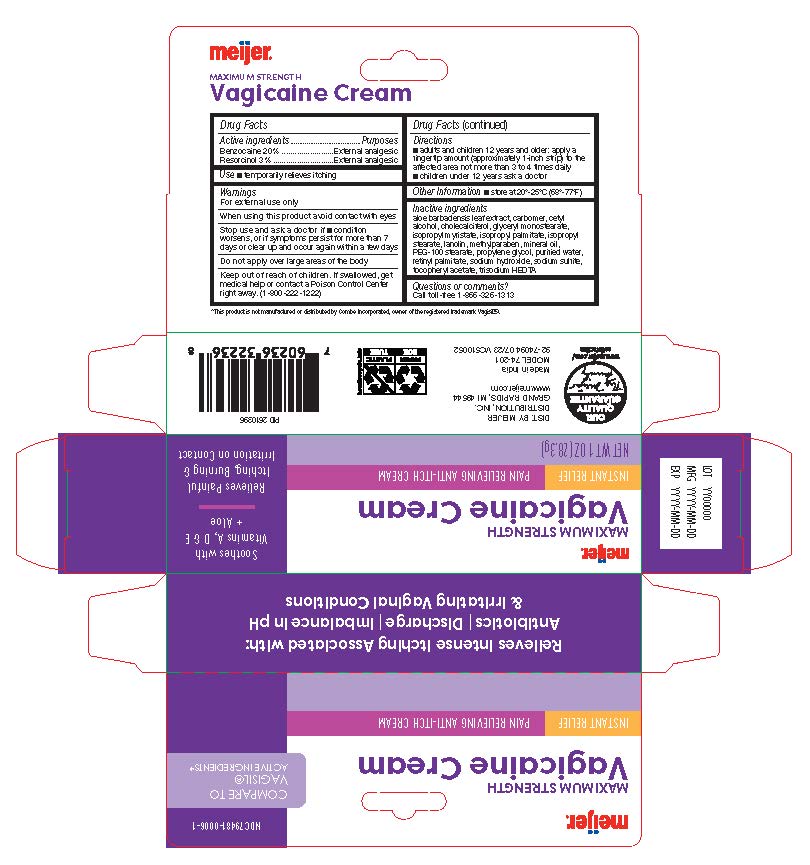
Label: MEIJER MAXIMUM STRENGTH VAGICAINE- benzocaine, resorcinol cream
- NDC Code(s): 79481-0006-1
- Packager: Meijer Distribution Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Use
- Warnings
- Other information
-
Inactive Ingredients
aloe barbadensis leaf extract, carbomer, cetyl alcohol, cholecalciferol, glyceryl monostearate, isopropyl myristate, isopropyl palmitate, isopropyl stearate, lanolin, methylparaben, mineral oil, PEG-100 stearate, propylene glycol, purified water, retinyl palmitate, sodium hydroxide, sodium sulfate, tocopheryl acetate, trisodium HEDTA
- Questions or comments
- Meijer Maximum Strength Vagicaine Cream
-
INGREDIENTS AND APPEARANCE
MEIJER MAXIMUM STRENGTH VAGICAINE
benzocaine, resorcinol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79481-0006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RESORCINOL (UNII: YUL4LO94HK) (RESORCINOL - UNII:YUL4LO94HK) RESORCINOL 0.84 g in 28 g BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 5.6 g in 28 g Inactive Ingredients Ingredient Name Strength CHOLECALCIFEROL (UNII: 1C6V77QF41) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) PEG-100 STEARATE (UNII: YD01N1999R) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYLPARABEN (UNII: A2I8C7HI9T) LANOLIN (UNII: 7EV65EAW6H) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) LIGHT MINERAL OIL (UNII: N6K5787QVP) CARBOMER 940 (UNII: 4Q93RCW27E) SODIUM METABISULFITE (UNII: 4VON5FNS3C) CETYL ALCOHOL (UNII: 936JST6JCN) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) ISOPROPYL STEARATE (UNII: 43253ZW1MZ) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) EDETATE DISODIUM (UNII: 7FLD91C86K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79481-0006-1 28.3 g in 1 TUBE; Type 0: Not a Combination Product 07/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 07/01/2023 Labeler - Meijer Distribution Inc (006959555) Establishment Name Address ID/FEI Business Operations ANICARE PHARMACEUTICALS PRIVATE LIMITED 916837425 manufacture(79481-0006)