Label: AMLODIPINE AND BENAZEPRIL HYDROCHLORIDE capsule
- NDC Code(s): 68788-8454-3
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 65862-586
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AMLODIPINE AND BENAZEPRIL HYDROCHLORIDE CAPSULES safely and effectively. See full prescribing information for AMLODIPINE AND ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: FETAL TOXICITY
When pregnancy is detected, discontinue amlodipine and benazepril hydrochloride as soon as possible [see Warnings and Precautions (5.1)].
Close
Drugs that act directly on the renin-angiotensin system (RAS) can cause injury and death to the developing fetus [see Warnings and Precautions (5.1)]. -
1 INDICATIONS AND USAGE1.1 Hypertension - Amlodipine and benazepril hydrochloride capsules are indicated for the treatment of hypertension in patients not adequately controlled on monotherapy with either agent.
-
2 DOSAGE AND ADMINISTRATION2.1 General Considerations - The recommended initial dose is amlodipine 2.5 mg and benazepril 10 mg orally once-daily. Begin therapy with amlodipine and benazepril hydrochloride capsules only ...
-
3 DOSAGE FORMS AND STRENGTHSAmlodipine and benazepril hydrochloride capsules USP are available as follows: 2.5 mg/10 mg, 5 mg/10 mg, 5 mg/20 mg, 5 mg/40 mg, 10 mg/20 mg, and 10 mg/40 mg.
-
4 CONTRAINDICATIONS• Do not coadminister aliskiren with angiotensin receptor blockers (ARBs), angiotensin-converting enzyme (ACE) inhibitors, including amlodipine and benazepril hydrochloride capsules in patients ...
-
5 WARNINGS AND PRECAUTIONS5.1 Fetal Toxicity - Amlodipine Benazepril hydrochloride can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Drug/Drug Interactions - Amlodipine - Simvastatin: Coadministration of simvastatin with amlodipine increases the systemic exposure of simvastatin. Limit the dose of simvastatin in patients ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Amlodipine and benazepril hydrochloride can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the RAS during the second and third ...
-
10 OVERDOSAGEOnly a few cases of human overdose with amlodipine have been reported. One patient was asymptomatic after a 250 mg ingestion; another, who combined 70 mg of amlodipine with an unknown large ...
-
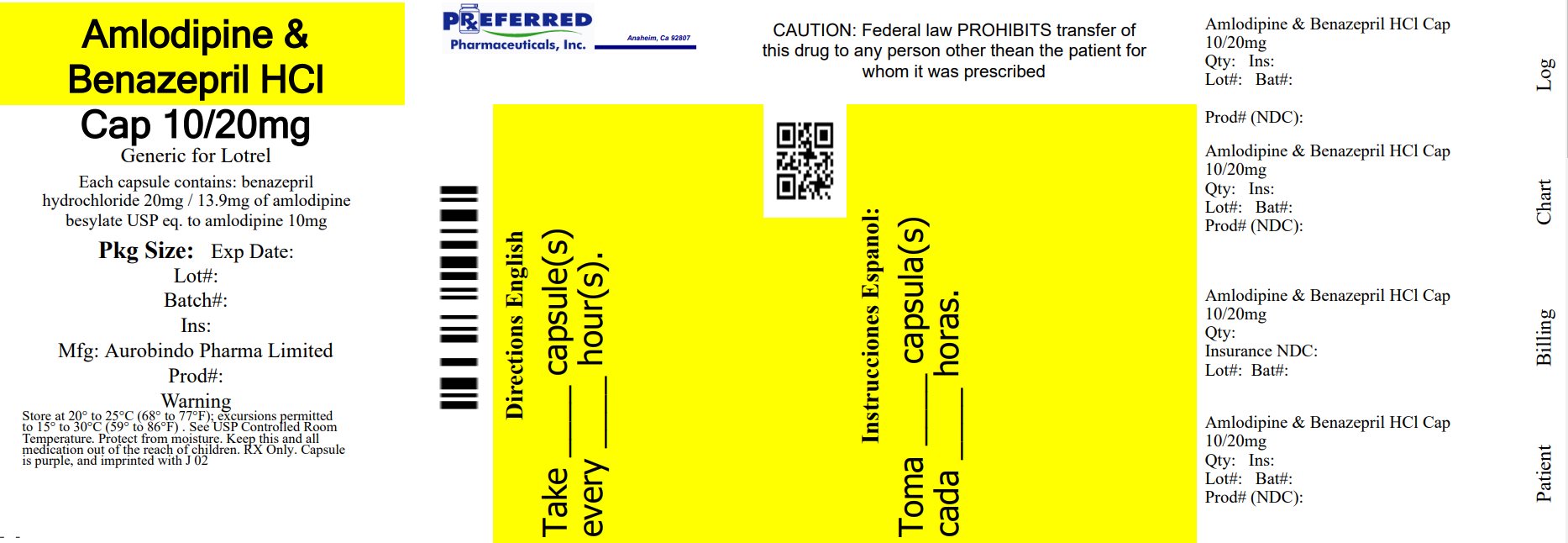
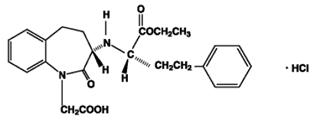
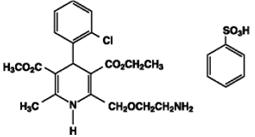
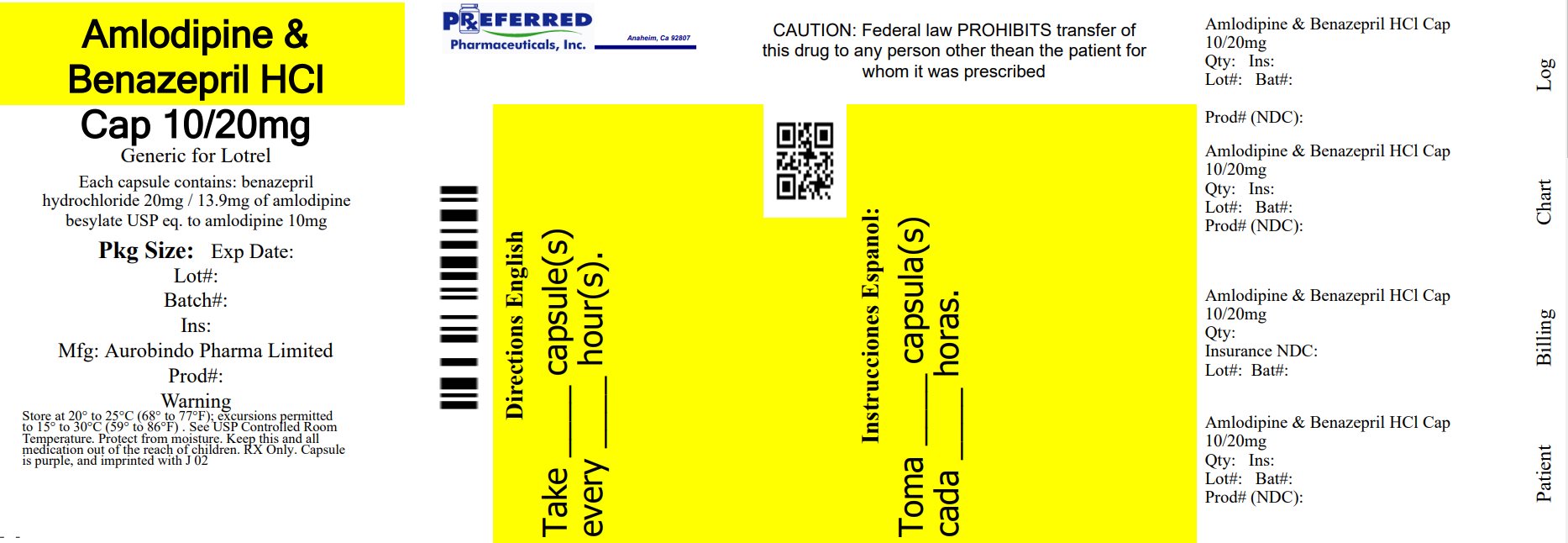
11 DESCRIPTIONAmlodipine and benazepril hydrochloride capsules USP are a combination of amlodipine besylate and benazepril hydrochloride. Benazepril hydrochloride USP is a white to off-white, crystalline ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Benazepril - Benazepril and benazeprilat inhibit ACE in human subjects and in animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity and mutagenicity studies have not been conducted with this combination. However, these studies have been conducted ...
-
14 CLINICAL STUDIESOver 950 patients received amlodipine and benazepril hydrochloride once-daily in 6 double-blind, placebo-controlled studies. The antihypertensive effect of a single dose persisted for 24 hours ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAmlodipine and benazepril hydrochloride is available as capsules containing amlodipine besylate USP (3.5 mg, 6.9 mg or 13.9 mg, equivalent to 2.5 mg, 5 mg or 10 mg of amlodipine respectively) ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Pregnancy: Advise female patients of childbearing age about the consequences of exposure to amlodipine and ...
-
Patient InformationAmlodipine and Benazepril Hydrochloride Capsules USP - (am loe' di peen and ben az' e pril hye" droe klor' ide) Read this Patient Information leaflet before you start taking amlodipine and ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg/20 mg (30 Capsules Bottle)NDC 68788-8454-3 - Rx only - Amlodipine and - Benazepril - Hydrochloride - Capsules USP - 10 mg*/20 mg - AUROBINDO Repackaged By: Preferred Pharmaceuticals Inc.
-
INGREDIENTS AND APPEARANCEProduct Information