Label: LORAZEPAM injection
- NDC Code(s): 65219-368-01, 65219-368-02
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only - For intravenous and intramuscular use NOT - FOR USE IN NEONATES CONTAINS - BENZYL ALCOHOL
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation (see WARNINGS and PRECAUTIONS).
- The use of benzodiazepines, including Lorazepam Injection, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing Lorazepam Injection and throughout treatment, assess each patient's risk for abuse, misuse, and addiction (see WARNINGS).
- The continued use of benzodiazepines for several days to weeks may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Although Lorazepam Injection is indicated only for intermittent use (see INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION), if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of Lorazepam Injection may precipitate acute withdrawal reactions, which can be life-threatening. For patients using Lorazepam Injection more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue Lorazepam Injection (see WARNINGS).
-
DESCRIPTION
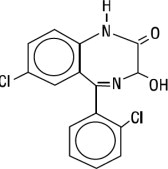
Lorazepam, a benzodiazepine with antianxiety, sedative, and anticonvulsant effects, is intended for the intramuscular or intravenous routes of administration. It has the chemical formula ...
Lorazepam, a benzodiazepine with antianxiety, sedative, and anticonvulsant effects, is intended for the intramuscular or intravenous routes of administration. It has the chemical formula: 7-chloro-5(2- chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1, 4-benzodiazepin-2-one. The molecular weight is 321.16, and the C.A.S. No. is [846-49-1]. The structural formula is:
Lorazepam is a nearly white powder almost insoluble in water. Each mL of sterile injection contains 2 mg of lorazepam, 0.18 mL polyethylene glycol 400 in propylene glycol with 2% benzyl alcohol as preservative.
Close -
CLINICAL PHARMACOLOGY
Lorazepam interacts with the γ-aminobutyric acid (GABA)-benzodiazepine receptor complex, which is widespread in the brain of humans as well as other species. This interaction is presumed to be ...
Lorazepam interacts with the γ-aminobutyric acid (GABA)-benzodiazepine receptor complex, which is widespread in the brain of humans as well as other species. This interaction is presumed to be responsible for lorazepam's mechanism of action. Lorazepam exhibits relatively high and specific affinity for its recognition site but does not displace GABA. Attachment to the specific binding site enhances the affinity of GABA for its receptor site on the same receptor complex. The pharmacodynamic consequences of benzodiazepine agonist actions include antianxiety effects, sedation, and reduction of seizure activity. The intensity of action is directly related to the degree of benzodiazepine receptor occupancy.
Effects in Pre-Operative Patients
Intravenous or intramuscular administration of the recommended dose of 2 mg to 4 mg of Lorazepam Injection to adult patients is followed by dose-related effects of sedation (sleepiness or drowsiness), relief of preoperative anxiety, and lack of recall of events related to the day of surgery in the majority of patients. The clinical sedation (sleepiness or drowsiness) thus noted is such that the majority of patients are able to respond to simple instructions whether they give the appearance of being awake or asleep. The lack of recall is relative rather than absolute, as determined under conditions of careful patient questioning and testing, using props designed to enhance recall. The majority of patients under these reinforced conditions had difficulty recalling perioperative events or recognizing props from before surgery. The lack of recall and recognition was optimum within 2 hours following intramuscular administration and 15 to 20 minutes after intravenous injection.
The intended effects of the recommended adult dose of Lorazepam Injection usually last 6 to 8 hours. In rare instances, and where patients received greater than the recommended dose, excessive sleepiness and prolonged lack of recall were noted. As with other benzodiazepines, unsteadiness, enhanced sensitivity to CNS-depressant effects of ethyl alcohol and other drugs were noted in isolated and rare cases for greater than 24 hours.
Physiologic Effects in Healthy Adults
Studies in healthy adult volunteers reveal that intravenous lorazepam in doses up to 3.5 mg/70 kg does not alter sensitivity to the respiratory stimulating effect of carbon dioxide and does not enhance the respiratory-depressant effects of doses of meperidine up to 100 mg/70 kg (also determined by carbon dioxide challenge) as long as patients remain sufficiently awake to undergo testing. Upper airway obstruction has been observed in rare instances where the patient received greater than the recommended dose and was excessively sleepy and difficult to arouse (see WARNINGS and ADVERSE REACTIONS).
Clinically employed doses of Lorazepam Injection do not greatly affect the circulatory system in the supine position or employing a 70-degree tilt test. Doses of 8 mg to 10 mg of intravenous lorazepam (2 to 2-1/2 times the maximum recommended dosage) will produce loss of lid reflexes within 15 minutes.
Studies in 6 healthy young adults who received lorazepam injection and no other drugs revealed that visual tracking (the ability to keep a moving line centered) was impaired for a mean of 8 hours following administration of 4 mg of intramuscular lorazepam and 4 hours following administration of 2 mg intramuscularly with considerable subject variation. Similar findings were noted with pentobarbital, 150 and 75 mg. Although this study showed that both lorazepam and pentobarbital interfered with eye-hand coordination, the data are insufficient to predict when it would be safe to operate a motor vehicle or engage in a hazardous occupation or sport.
Pharmacokinetics and Metabolism
ABSORPTION
Intravenous
A 4-mg dose provides an initial concentration of approximately 70 ng/mL.
Intramuscular
Following intramuscular administration, lorazepam is completely and rapidly absorbed reaching peak concentrations within 3 hours. A 4-mg dose provides a Cmax of approximately 48 ng/mL. Following administration of 1.5 to 5 mg of lorazepam intramuscular, the amount of lorazepam delivered to the circulation is proportional to the dose administered.
DISTRIBUTION/METABOLISM/ELIMINATION
At clinically relevant concentrations, lorazepam is 91±2% bound to plasma proteins; its volume of distribution is approximately 1.3 L/kg. Unbound lorazepam penetrates the blood/brain barrier freely by passive diffusion, a fact confirmed by CSF sampling. Following parenteral administration, the terminal half- life and total clearance averaged 14±5 hours and 1.1±0.4 mL/min/kg, respectively.
Lorazepam is extensively conjugated to the 3-O-phenolic glucuronide in the liver and is known to undergo enterohepatic recirculation. Lorazepam glucuronide is an inactive metabolite and is eliminated mainly by the kidneys.
Following a single 2-mg oral dose of 14C-lorazepam to 8 healthy subjects, 88±4% of the administered dose was recovered in urine and 7±2% was recovered in feces. The percent of administered dose recovered in urine as lorazepam glucuronide was 74±4%. Only 0.3% of the dose was recovered as unchanged lorazepam, and the remainder of the radioactivity represented minor metabolites.
Special Populations
EFFECT OF AGE
Pediatrics
NEONATES (BIRTH TO 1 MONTH)
Following a single 0.05 mg/kg (n=4) or 0.1 mg/kg (n=6) intravenous dose of lorazepam, mean total clearance normalized to body weight was reduced by 80% compared to normal adults, terminal half-life was prolonged 3-fold, and volume of distribution was decreased by 40% in neonates with asphyxia neonatorum compared to normal adults. All neonates were of ≥37 weeks of gestational age.
INFANTS (1 MONTH UP TO 2 YEARS)
There is no information on the pharmacokinetic profile of lorazepam in infants in the age range of 1 month to 2 years.
CHILDREN (2 YEARS TO 12 YEARS)
Total (bound and unbound) lorazepam had a 50% higher mean volume of distribution (normalized to body-weight) and a 30% longer mean half-life in children with acute lymphocytic leukemia in complete remission (2 to 12 years, n=37) compared to normal adults (n=10). Unbound lorazepam clearance normalized to body-weight was comparable in children and adults.
ADOLESCENTS (12 YEARS TO 18 YEARS)
Total (bound and unbound) lorazepam had a 50% higher mean volume of distribution (normalized to body-weight) and a mean half-life that was two fold greater in adolescents with acute lymphocytic leukemia in complete remission (12 to 18 years, n=13) compared to normal adults (n=10). Unbound lorazepam clearance normalized to body-weight was comparable in adolescents and adults.
Elderly
Following single intravenous doses of 1.5 to 3 mg of Lorazepam Injection, mean total body clearance of lorazepam decreased by 20% in 15 elderly subjects of 60 to 84 years of age compared to that in 15 younger subjects of 19 to 38 years of age. Consequently, no dosage adjustment appears to be necessary in elderly subjects based solely on their age.
EFFECT OF RACE
Young Americans (n=15) and Japanese subjects (n=7) had very comparable mean total clearance value of 1 mL/min/kg. However, elderly Japanese subjects had a 20% lower mean total clearance than elderly Americans, 0.59 mL/min/kg vs 0.77 mL/min/kg, respectively.
PATIENTS WITH RENAL INSUFFICIENCY
Because the kidney is the primary route of elimination of lorazepam glucuronide, renal impairment would be expected to compromise its clearance. This should have no direct effect on the glucuronidation (and inactivation) of lorazepam. There is a possibility that the enterohepatic circulation of lorazepam glucuronide leads to a reduced efficiency of the net clearance of lorazepam in this population.
Six normal subjects, six patients with renal impairment (Clcr of 22±9 mL/min), and four patients on chronic maintenance hemodialysis were given single 1.5 to 3 mg intravenous doses of lorazepam. Mean volume of distribution and terminal half-life values of lorazepam were 40% and 25% higher, respectively, in renally impaired patients than in normal subjects. Both parameters were 75% higher in patients undergoing hemodialysis than in normal subjects. Overall, though, in this group of subjects the mean total clearance of lorazepam did not change. About 8% of the administered intravenous dose was removed as intact lorazepam during the 6-hour dialysis session.
The kinetics of lorazepam glucuronide were markedly affected by renal dysfunction. The mean terminal half-life was prolonged by 55% and 125% in renally impaired patients and patients under hemodialysis, respectively, as compared to normal subjects. The mean metabolic clearance decreased by 75% and 90% in renally impaired patients and patients under hemodialysis, respectively, as compared with normal subjects.
About 40% of the administered lorazepam intravenous dose was removed as glucuronide conjugate during the 6-hour dialysis session.
HEPATIC DISEASE
Because cytochrome oxidation is not involved with the metabolism of lorazepam, liver disease would not be expected to have an effect on metabolic clearance. This prediction is supported by the observation that following a single 2 mg intravenous dose of lorazepam, cirrhotic male patients (n=13) and normal male subjects (n=11) exhibited no substantive difference in their ability to clear lorazepam.
EFFECT OF SMOKING
Administration of a single 2 mg intravenous dose of lorazepam showed that there was no difference in any of the pharmacokinetic parameters of lorazepam between cigarette smokers (n=10, mean=31 cigarettes per day) and nonsmoking subjects (n=10) who were matched for age, weight and gender.
CloseClinical Studies
The effectiveness of Lorazepam Injection in status epilepticus was established in two multi-center controlled trials in 177 patients. With rare exceptions, patients were between 18 and 65 years of age. More than half the patients in each study had tonic-clonic status epilepticus; patients with simple partial and complex partial status epilepticus comprised the rest of the population studied, along with a smaller number of patients who had absence status.
One study (n=58) was a double-blind active-control trial comparing Lorazepam Injection and diazepam. Patients were randomized to receive Lorazepam Injection 2 mg intravenous (with an additional 2 mg intravenous if needed) or diazepam 5 mg intravenous (with an additional 5 mg intravenous if needed). The primary outcome measure was a comparison of the proportion of responders in each treatment group, where a responder was defined as a patient whose seizures stopped within 10 minutes after treatment and who continued seizure-free for at least an additional 30 minutes. Twenty-four of the 30 (80%) patients were deemed responders to Lorazepam Injection and 16/28 (57%) patients were deemed responders to diazepam (p=0.04). Of the 24 Lorazepam Injection responders, 23 received both 2 mg infusions.
Non-responders to Lorazepam Injection 4 mg were given an additional 2 to 4 mg Lorazepam Injection; non-responders to diazepam 10 mg were given an additional 5 to 10 mg diazepam. After this additional dose administration, 28/30 (93%) of patients randomized to Lorazepam Injection and 24/28 (86%) of patients randomized to diazepam were deemed responders, a difference that was not statistically significant.
Although this study provides support for the efficacy of Lorazepam Injection as the treatment for status epilepticus, it cannot speak reliably or meaningfully to the comparative performance of either diazepam (Valium) or Lorazepam Injection under the conditions of actual use.
A second study (n=119) was a double-blind dose-comparison trial with 3 doses of Lorazepam Injection: 1 mg, 2 mg, and 4 mg. Patients were randomized to receive one of the three doses of Lorazepam Injection. The primary outcome and definition of responder were as in the first study. Twenty-five of 41 patients (61%) responded to 1 mg Lorazepam Injection; 21/37 patients (57%) responded to 2 mg Lorazepam Injection; and 31/41 (76%) responded to 4 mg Lorazepam Injection. The p-value for a statistical test of the difference between the Lorazepam Injection 4 mg dose group and the Lorazepam Injection 1-mg dose group was 0.08 (two-sided). Data from all randomized patients were used in this test.
Although analyses failed to detect an effect of age, sex, or race on the effectiveness of Lorazepam Injection in status epilepticus, the numbers of patients evaluated were too few to allow a definitive conclusion about the role these factors may play.
-
INDICATIONS AND USAGE
Status Epilepticus - Lorazepam Injection is indicated for the treatment of status epilepticus. Preanesthetic - Lorazepam Injection is indicated in adult patients for preanesthetic ...Close
Preanesthetic
Lorazepam Injection is indicated in adult patients for preanesthetic medication, producing sedation (sleepiness or drowsiness), relief of anxiety, and a decreased ability to recall events related to the day of surgery. It is most useful in those patients who are anxious about their surgical procedure and who would prefer to have diminished recall of the events of the day of surgery (see PRECAUTIONS, Information for Patients).
-
CONTRAINDICATIONS
Lorazepam Injection is contraindicated in patients with a known sensitivity to benzodiazepines or its vehicle (polyethylene glycol, propylene glycol, and benzyl alcohol), in patients with acute ...
Lorazepam Injection is contraindicated in patients with a known sensitivity to benzodiazepines or its vehicle (polyethylene glycol, propylene glycol, and benzyl alcohol), in patients with acute narrow-angle glaucoma, or in patients with sleep apnea syndrome. It is also contraindicated in patients with severe respiratory insufficiency, except in those patients requiring relief of anxiety and/or diminished recall of events while being mechanically ventilated. The use of Lorazepam Injection intra-arterially is contraindicated because, as with other injectable benzodiazepines, inadvertent intra-arterial injection may produce arteriospasm resulting in gangrene which may require amputation (see WARNINGS).
Lorazepam Injection is contraindicated for use in premature infants because the formulation contains benzyl alcohol (see WARNINGS and PRECAUTIONS, Pediatric Use).
Close -
WARNINGS
Risks from Concomitant Use with Opioids - Concomitant use of benzodiazepines, including Lorazepam Injection, and opioids may result in profound sedation, respiratory depression, coma, and death ...
Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including Lorazepam Injection, and opioids may result in profound sedation, respiratory depression, coma, and death. If a decision is made to use Lorazepam Injection concomitantly with opioids, monitor patients closely for respiratory depression and sedation (see PRECAUTIONS, Drug Interactions).
Abuse, Misuse, and Addiction
The use of benzodiazepines, including Lorazepam Injection, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see DRUG ABUSE AND DEPENDENCE, Abuse).
Before prescribing Lorazepam Injection and throughout treatment, assess each patient's risk for abuse, misuse, and addiction. Use of Lorazepam Injection, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of Lorazepam Injection along with monitoring for signs and symptoms of abuse, misuse, and addiction. Do not exceed the recommended dosing frequency; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal Reactions After Use of Lorazepam Injection More Frequently Than Recommended
For patients using Lorazepam Injection more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue Lorazepam Injection (a patient-specific plan should be used to taper the dose).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
ACUTE WITHDRAWAL REACTIONS
The continued use of benzodiazepines may lead to clinically significant physical dependence. Although Lorazepam Injection is indicated only for intermittent use (see INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION), if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of Lorazepam Injection, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see DRUG ABUSE AND DEPENDENCE, Dependence).
PROTRACTED WITHDRAWAL SYNDROME
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see DRUG ABUSE AND DEPENDENCE, Dependence).
Use in Status Epilepticus
MANAGEMENT OF STATUS EPILEPTICUS
Status epilepticus is a potentially life-threatening condition associated with a high risk of permanent neurological impairment, if inadequately treated. The treatment of status, however, requires far more than the administration of an anticonvulsant agent. It involves observation and management of all parameters critical to maintaining vital function and the capacity to provide support of those functions as required. Ventilatory support must be readily available. The use of benzodiazepines, like Lorazepam Injection, is ordinarily only one step of a complex and sustained intervention which may require additional interventions (e.g., concomitant intravenous administration of phenytoin). Because status epilepticus may result from a correctable acute cause such as hypoglycemia, hyponatremia, or other metabolic or toxic derangement, such an abnormality must be immediately sought and corrected. Furthermore, patients who are susceptible to further seizure episodes should receive adequate maintenance antiepileptic therapy.
Any healthcare professional who intends to treat a patient with status epilepticus should be familiar with this package insert and the pertinent medical literature concerning current concepts for the treatment of status epilepticus. A comprehensive review of the considerations critical to the informed and prudent management of status epilepticus cannot be provided in drug product labeling. The archival medical literature contains many informative references on the management of status epilepticus, among them the report of the working group on status epilepticus of the Epilepsy Foundation of America “Treatment of Convulsive Status Epilepticus” (JAMA 1993; 270:854-859). As noted in the report just cited, it may be useful to consult with a neurologist if a patient fails to respond (e.g., fails to regain consciousness).
For the treatment of status epilepticus, the usual recommended dose of Lorazepam Injection is 4 mg given slowly (2 mg/min) for patients 18 years and older. If seizures cease, no additional Lorazepam Injection is required. If seizures continue or recur after a 10- to 15-minute observation period, an additional 4 mg intravenous dose may be slowly administered. Experience with further doses of Lorazepam Injection is very limited. The usual precautions in treating status epilepticus should be employed. An intravenous infusion should be started, vital signs should be monitored, an unobstructed airway should be maintained, and artificial ventilation equipment should be available.
Preanesthetic Use
AIRWAY OBSTRUCTION MAY OCCUR IN HEAVILY SEDATED PATIENTS. INTRAVENOUS LORAZEPAM AT ANY DOSE, WHEN GIVEN EITHER ALONE OR IN COMBINATION WITH OTHER DRUGS ADMINISTERED DURING ANESTHESIA, MAY PRODUCE HEAVY SEDATION; THEREFORE, EQUIPMENT NECESSARY TO MAINTAIN A PATENT AIRWAY AND TO SUPPORT RESPIRATION/VENTILATION SHOULD BE AVAILABLE.
As is true of similar CNS-acting drugs, the decision as to when patients who have received injectable lorazepam, particularly on an outpatient basis, may again operate machinery, drive a motor vehicle, or engage in hazardous or other activities requiring attention and coordination must be individualized. It is recommended that no patient engage in such activities for a period of 24 to 48 hours or until the effects of the drug, such as drowsiness, have subsided, whichever is longer. Impairment of performance may persist for greater intervals because of extremes of age, concomitant use of other drugs, stress of surgery, or the general condition of the patient.
Clinical trials have shown that patients over the age of 50 years may have a more profound and prolonged sedation with intravenous lorazepam (see DOSAGE AND ADMINISTRATION, Preanesthetic).
As with all central-nervous-system-depressant drugs, care should be exercised in patients given injectable lorazepam as premature ambulation may result in injury from falling.
There is no added beneficial effect from the addition of scopolamine to injectable lorazepam, and their combined effect may result in an increased incidence of sedation, hallucination and irrational behavior.
General (All Uses)
PRIOR TO INTRAVENOUS USE, LORAZEPAM INJECTION MUST BE DILUTED WITH AN EQUAL AMOUNT OF COMPATIBLE DILUENT (see DOSAGE AND ADMINISTRATION). INTRAVENOUS INJECTION SHOULD BE MADE SLOWLY AND WITH REPEATED ASPIRATION. CARE SHOULD BE TAKEN TO DETERMINE THAT ANY INJECTION WILL NOT BE INTRA-ARTERIAL AND THAT PERIVASCULAR EXTRAVASATION WILL NOT TAKE PLACE. IN THE EVENT THAT A PATIENT COMPLAINS OF PAIN DURING INTENDED INTRAVENOUS INJECTION OF LORAZEPAM INJECTION, THE INJECTION SHOULD BE STOPPED IMMEDIATELY TO DETERMINE IF INTRA-ARTERIAL INJECTION OR PERIVASCULAR EXTRAVASATION HAS TAKEN PLACE.
Since the liver is the most likely site of conjugation of lorazepam and since excretion of conjugated lorazepam (glucuronide) is a renal function, this drug is not recommended for use in patients with hepatic and/or renal failure. Lorazepam Injection should be used with caution in patients with mild-to-moderate hepatic or renal disease (see DOSAGE AND ADMINISTRATION).
Neonatal Sedation and Withdrawal Syndrome
Use of Lorazepam Injection late in pregnancy can result in sedation (respiratory depression. lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate (see PRECAUTIONS, Pregnancy). Monitor neonates exposed to Lorazepam Injection during pregnancy or labor for signs of sedation and monitor neonates exposed to Lorazepam Injection during pregnancy for signs of withdrawal; manage these neonates accordingly.
Usage in Preterm Infants and Neonates
Lorazepam Injection contains benzyl alcohol. Exposure to excessive amounts of benzyl alcohol has been associated with toxicity (hypotension, metabolic acidosis), particularly in neonates, and an increased incidence of kernicterus, particularly in small preterm infants. There have been rare reports of deaths, primarily in preterm infants, associated with exposure to excessive amounts of benzyl alcohol. The amount of benzyl alcohol from medications is usually considered negligible compared to that received in flush solutions containing benzyl alcohol. Administration of high dosages of medications (including Lorazepam Injection) containing this preservative must take into account the total amount of benzyl alcohol administered. The recommended dosage range of Lorazepam Injection for preterm and term infants includes amounts of benzyl alcohol well below that associated with toxicity; however, the amount of benzyl alcohol at which toxicity may occur is not known. If the patient requires more than the recommended dosages or other medications containing this preservative, the practitioner must consider the daily metabolic load of benzyl alcohol from these combined sources (see WARNINGS and PRECAUTIONS, Pediatric Use).
Pediatric Neurotoxicity
Published animal studies demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity increase neuronal apoptosis in the developing brain and result in long-term cognitive deficits when used for longer than 3 hours. The clinical significance of these findings is not clear. However, based on the available data, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but may extend out to approximately three years of age in humans (see PRECAUTIONS, Pregnancy, Pediatric Use; ANIMAL TOXICOLOGY AND/OR PHARMACOLOGY).
Some published studies in children suggest that similar deficits may occur after repeated or prolonged exposures to anesthetic agents early in life and may result in adverse cognitive or behavioral effects. These studies have substantial limitations, and it is not clear if the observed effects are due to the anesthetic/sedation drug administration or other factors such as the surgery or underlying illness.
Anesthetic and sedation drugs are a necessary part of the care of children needing surgery, other procedures, or tests that cannot be delayed, and no specific medications have been shown to be safer than any other.
Decisions regarding the timing of any elective procedures requiring anesthesia should take into consideration the benefits of the procedure weighed against the potential risks.
CloseEndoscopic Procedures
There are insufficient data to support the use of Lorazepam Injection for outpatient endoscopic procedures. Inpatient endoscopic procedures require adequate recovery room observation time.
When Lorazepam Injection is used for peroral endoscopic procedures, adequate topical or regional anesthesia is recommended to minimize reflex activity associated with such procedures.
-
PRECAUTIONS
General - The additive central-nervous-system effects of other drugs, such as phenothiazines, narcotic analgesics, barbiturates, antidepressants, scopolamine, and monoamine-oxidase inhibitors ...
General
The additive central-nervous-system effects of other drugs, such as phenothiazines, narcotic analgesics, barbiturates, antidepressants, scopolamine, and monoamine-oxidase inhibitors, should be borne in mind when these other drugs are used concomitantly with or during the period of recovery from Lorazepam Injection (see CLINICAL PHARMACOLOGY and WARNINGS).
Extreme caution must be used when administering Lorazepam Injection to elderly patients, very ill patients, or to patients with limited pulmonary reserve because of the possibility that hypoventilation and/or hypoxic cardiac arrest may occur. Resuscitative equipment for ventilatory support should be readily available (see WARNINGS and DOSAGE AND ADMINISTRATION).
When lorazepam injection is used intravenous as the premedicant prior to regional or local anesthesia, the possibility of excessive sleepiness or drowsiness may interfere with patient cooperation in determining levels of anesthesia. This is most likely to occur when greater than 0.05 mg/kg is given and when narcotic analgesics are used concomitantly with the recommended dose (see ADVERSE REACTIONS).
As with all benzodiazepines, paradoxical reactions may occur in rare instances and in an unpredictable fashion (see ADVERSE REACTIONS). In these instances, further use of the drug in these patients should be considered with caution.
There have been reports of possible propylene glycol toxicity (e.g., lactic acidosis, hyperosmolality, hypotension) and possible polyethylene glycol toxicity (e.g., acute tubular necrosis) during administration of Lorazepam Injection at higher than recommended doses. Symptoms may be more likely to develop in patients with renal impairment.
Information for Patients
RISKS FROM CONCOMITANT USE WITH OPIOIDS
Concomitant use of benzodiazepines, including Lorazepam Injection, and opioids may result in profound sedation, respiratory depression, coma, and death (see WARNINGS and PRECAUTIONS, Drug Interactions).
ABUSE, MISUSE, AND ADDICTION
Inform patients that the use of Lorazepam Injection more frequently than recommended, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug (see WARNINGS and DRUG ABUSE AND DEPENDENCE).
WITHDRAWAL REACTIONS
Inform patients that use of Lorazepam Injection more frequently than recommended may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of Lorazepam Injection may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see WARNINGS and DRUG ABUSE AND DEPENDENCE).
EXCESSIVE SEDATION
Patients should be informed of the pharmacological effects of the drug, including sedation, relief of anxiety, and lack of recall, the duration of these effects (about 8 hours), and be apprised of the risks as well as the benefits of therapy.
Patients who receive Lorazepam Injection as a premedicant should be cautioned that driving a motor vehicle, operating machinery, or engaging in hazardous or other activities requiring attention and coordination, should be delayed for 24 to 48 hours following the injection or until the effects of the drug, such as drowsiness, have subsided, whichever is longer. Sedatives, tranquilizers and narcotic analgesics may produce a more prolonged and profound effect when administered along with injectable Lorazepam Injection. This effect may take the form of excessive sleepiness or drowsiness and, on rare occasions, interfere with recall and recognition of events of the day of surgery and the day after.
Patients should be advised that getting out of bed unassisted may result in falling and injury if undertaken within 8 hours of receiving lorazepam injection. Since tolerance for CNS depressants will be diminished in the presence of Lorazepam Injection, these substances should either be avoided or taken in reduced dosage. Alcoholic beverages should not be consumed for at least 24 to 48 hours after receiving lorazepam injectable due to the additive effects on central-nervous-system depression seen with benzodiazepines in general.
Elderly patients should be told that Lorazepam Injection may make them very sleepy for a period longer than 6 to 8 hours following surgery.
EFFECT OF ANESTHETIC AND SEDATION DRUGS ON EARLY BRAIN DEVELOPMENT
Studies conducted in young animals and children suggest repeated or prolonged use of general anesthetic or sedation drugs in children younger than 3 years may have negative effects on their developing brains.
Discuss with parents and caregivers the benefits, risks, and timing and duration of surgery or procedures requiring anesthetic and sedation drugs (see WARNINGS, Pediatric Neurotoxicity).
PREGNANCY
Advise pregnant females who are administered Lorazepam Injection late in pregnancy that Lorazepam Injection can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns (see WARNINGS, Neonatal Sedation and Withdrawal Syndrome and PRECAUTIONS, Pregnancy). Instruct patients to inform their healthcare provider if they are pregnant.
NURSING
Instruct patients to notify their healthcare provider if they are breastfeeding or intend to breastfeed. Instruct breastfeeding patients who are administered Lorazepam Injection to monitor infants for excessive sedation, poor feeding and poor weight gain, and to seek medical attention if they notice these signs (see PRECAUTIONS, Nursing Mothers).
Laboratory Tests
In clinical trials, no laboratory test abnormalities were identified with either single or multiple doses of Lorazepam Injection. These tests included: CBC, urinalysis, SGOT, SGPT, bilirubin, alkaline phosphatase, LDH, cholesterol, uric acid, BUN, glucose, calcium, phosphorus, and total proteins.
Drug Interactions
INTERACTION WITH BENZODIAZEPINES AND OTHER CNS DEPRESSANTS
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Monitor patients closely for respiratory depression and sedation.
Lorazepam Injection, like other injectable benzodiazepines, produces additive depression of the central nervous system when administered with other CNS depressants such as ethyl alcohol, phenothiazines, barbiturates, MAO inhibitors, and other antidepressants.
When scopolamine is used concomitantly with injectable lorazepam, an increased incidence of sedation, hallucinations and irrational behavior has been observed.
There have been rare reports of significant respiratory depression, stupor and/or hypotension with the concomitant use of loxapine and lorazepam.
Marked sedation, excessive salivation, ataxia, and, rarely, death have been reported with the concomitant use of clozapine and lorazepam.
Apnea, coma, bradycardia, arrhythmia, heart arrest, and death have been reported with the concomitant use of haloperidol and lorazepam.
The risk of using lorazepam in combination with scopolamine, loxapine, clozapine, haloperidol, or other CNS-depressant drugs has not been systematically evaluated. Therefore, caution is advised if the concomitant administration of lorazepam and these drugs is required.
Concurrent administration of any of the following drugs with lorazepam had no effect on the pharmacokinetics of lorazepam: metoprolol, cimetidine, ranitidine, disulfiram, propranolol, metronidazole, and propoxyphene. No change in Lorazepam Injection dosage is necessary when concomitantly given with any of these drugs.
LORAZEPAM-VALPROATE INTERACTION
Concurrent administration of lorazepam (2 mg intravenously) with valproate (250 mg twice daily orally for 3 days) to 6 healthy male subjects resulted in decreased total clearance of lorazepam by 40% and decreased formation rate of lorazepam glucuronide by 55%, as compared with lorazepam administered alone.
Accordingly, lorazepam plasma concentrations were about two-fold higher for at least 12 hours post-dose administration during valproate treatment. Lorazepam dosage should be reduced to 50% of the normal adult dose when this drug combination is prescribed in patients (see DOSAGE AND ADMINISTRATION).
LORAZEPAM-ORAL CONTRACEPTIVE STEROIDS INTERACTION
Coadministration of lorazepam (2 mg intravenously) with oral contraceptive steroids (norethindrone acetate, 1 mg, and ethinyl estradiol, 50 μg, for at least 6 months) to healthy females (n=7) was associated with a 55% decrease in half-life, a 50% increase in the volume of distribution, thereby resulting in an almost 3.7-fold increase in total clearance of lorazepam as compared with control healthy females (n=8). It may be necessary to increase the dose of Lorazepam Injection in female patients who are concomitantly taking oral contraceptives (see DOSAGE AND ADMINISTRATION).
LORAZEPAM-PROBENECID INTERACTION
Concurrent administration of lorazepam (2 mg intravenously) with probenecid (500 mg orally every 6 hours) to 9 healthy volunteers resulted in a prolongation of lorazepam half-life by 130% and a decrease in its total clearance by 45%. No change in volume of distribution was noted during probenecid co-treatment. Lorazepam Injection dosage needs to be reduced by 50% when coadministered with probenecid (see DOSAGE AND ADMINISTRATION).
Drug/Laboratory Test Interactions
No laboratory test abnormalities were identified when lorazepam was given alone or concomitantly with another drug, such as narcotic analgesics, inhalation anesthetics, scopolamine, atropine, and a variety of tranquilizing agents.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of carcinogenic potential emerged in rats and mice during an 18-month study with oral lorazepam. No studies regarding mutagenesis have been performed. The results of a preimplantation study in rats, in which the oral lorazepam dose was 20 mg/kg, showed no impairment of fertility.
Pregnancy
Risk Summary
Neonates born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal (see WARNINGS, Neonatal Sedation and Withdrawal Syndrome and Clinical Considerations). Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and of miscarriage in clinically recognizable pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia and sedation in neonates. Monitor neonates exposed to Lorazepam Injection during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems. Monitor neonates exposed to Lorazepam Injection during pregnancy for signs of withdrawal. Manage these neonates accordingly (see WARNINGS, Neonatal Sedation and Withdrawal Syndrome).
Data
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol. tobacco and other medications, have not confirmed these findings.
Animal Data
Reproductive studies in animals were performed in mice, rats, and two strains of rabbits. Occasional anomalies (reduction of tarsals, tibia, metatarsals, malrotated limbs, gastroschisis, malformed skull, and microphthalmia) were seen in drug-treated rabbits without relationship to dosage. Although all of these anomalies were not present in the concurrent control group, they have been reported to occur randomly in historical controls. At doses of 40 mg/kg orally or 4 mg/kg intravenously and higher, there was evidence of fetal resorption and increased fetal loss in rabbits which was not seen al lower doses.
Nursing Mothers
RISK SUMMARY
Lorazepam is present in breast milk. There are reports of sedation. poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. The effects of lorazepam on milk production are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Lorazepam Injection and any potential adverse effects on the breastfed infant from Lorazepam Injection or from the underlying maternal condition.
Clinical Considerations
Infants exposed to Lorazepam injection through breast milk should be monitored for sedation, poor feeding and poor weight gain.
Published studies in pregnant primates demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity during the period of peak brain development increases neuronal apoptosis in the developing brain of the offspring when used for longer than 3 hours. There are no data on pregnancy exposures in primates corresponding to periods prior to the third trimester in humans.
In a published study in primates, administration of an anesthetic dose of ketamine for 24 hours on Gestation Day 122 increased neuronal apoptosis in the developing brain of the fetus. In other published studies, administration of either isoflurane or propofol for 5 hours on Gestation Day 120 resulted in increased neuronal and oligodendrocyte apoptosis in the developing brain of the offspring. With respect to brain development, this time period corresponds to the third trimester of gestation in the human. The clinical significance of these findings is not clear; however, studies in juvenile animals suggest neuroapoptosis correlates with long-term cognitive deficits (see WARNINGS, Pediatric Neurotoxicity, Pediatric Use, and ANIMAL TOXICOLOGY AND/OR PHARMACOLOGY).
Pediatric Use
STATUS EPILEPTICUS
The safety and effectiveness of Lorazepam Injection for status epilepticus have not been established in pediatric patients. A randomized, double-blind, superiority-design clinical trial of Lorazepam Injection versus intravenous diazepam in 273 pediatric patients ages 3 months to 17 years failed to establish the efficacy of Lorazepam Injection for the treatment of status epilepticus. In that trial, assisted ventilation was required in 18% of patients treated with Lorazepam Injection versus 16% of patients treated with diazepam. Patients treated with Lorazepam Injection were also more likely to be reported as sedated (67% for Lorazepam Injection vs. 50% for diazepam), and the time for return to baseline mental status was, on average, 2 hours longer for Lorazepam Injection than for diazepam.
Open-label studies described in the medical literature included 273 pediatric patients; the age range was from a few hours old to 18 years of age. Paradoxical excitation was observed in 10% to 30% of the pediatric patients under 8 years of age and was characterized by tremors, agitation, euphoria, logorrhea, and brief episodes of visual hallucinations. Paradoxical excitation in pediatric patients also has been reported with other benzodiazepines when used for status epilepticus, as an anesthesia, or for pre-chemotherapy treatment.
Pediatric patients (as well as adults) with atypical petit mal status epilepticus have developed brief tonic-clonic seizures shortly after Lorazepam Injection was given. This “paradoxical” effect was also reported for diazepam and clonazepam. Nevertheless, the development of seizures after treatment with benzodiazepines is probably rare, based on the incidence in the uncontrolled treatment series reported (i.e., seizures were not observed for 112 pediatric patients and 18 adults or during approximately 400 doses).
Lorazepam Injection contains benzyl alcohol as a preservative. Benzyl alcohol, a component of this product, has been associated with serious adverse events and death, particularly in pediatric patients. The “gasping syndrome”, (characterized by central nervous system depression, metabolic acidosis, gasping respirations, and high levels of benzyl alcohol and its metabolites found in the blood and urine) has been associated with benzyl alcohol dosages greater than 99 mg/kg/day in neonates and low-birth-weight neonates. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Although normal therapeutic doses of this product deliver amounts of benzyl alcohol that are substantially lower than those reported in association with the “gasping syndrome”, the minimum amount of benzyl alcohol at which toxicity may occur is not known. Premature and low-birth-weight infants, as well as patients receiving high dosages, may be more likely to develop toxicity. Practitioners administering this and other medications containing benzyl alcohol should consider the combined daily metabolic load of benzyl alcohol from all sources.
PREANESTHETIC
There are insufficient data to support the efficacy of injectable lorazepam as a preanesthetic agent in patients less than 18 years of age.
GENERAL
Seizure activity and myoclonus have been reported to occur following administration of Lorazepam Injection, especially in very low birth weight neonates.
Pediatric patients may exhibit a sensitivity to benzyl alcohol, polyethylene glycol and propylene glycol, components of Lorazepam Injection (see CONTRAINDICATIONS). The “gasping syndrome”, characterized by central nervous system depression, metabolic acidosis, gasping respirations, and high levels of benzyl alcohol and its metabolites found in the blood and urine, has been associated with the administration of intravenous solutions containing the preservative benzyl alcohol in neonates. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Central nervous system toxicity, including seizures and intraventricular hemorrhage, as well as unresponsiveness, tachypnea, tachycardia, and diaphoresis have been associated with propylene glycol toxicity. Although normal therapeutic doses of Lorazepam Injection contain very small amounts of these compounds, premature and low-birth-weight infants as well as pediatric patients receiving high doses may be more susceptible to their effects.
Published juvenile animal studies demonstrate that the administration of anesthetic and sedation drugs, such as Lorazepam Injection that either block NMDA receptors or potentiate the activity of GABA during the period of rapid brain growth or synaptogenesis, results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of ketamine that produced a light surgical plane of anesthesia did not increase neuronal cell loss, however, treatment regimens of 5 hours or longer of isoflurane increased neuronal cell loss. Data from isoflurane-treated rodents and ketamine-treated primates suggest that the neuronal and oligodendrocyte cell losses are associated with prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in pregnant women, neonates and young children who require procedures with the potential risks suggested by the nonclinical data. (see WARNINGS, Pediatric Neurotoxicity; PRECAUTIONS, Pregnancy; ANIMAL TOXICOLOGY AND/OR PHARMACOLOGY).
CloseGeriatric Use
Clinical studies of Lorazepam Injection generally were not adequate to determine whether subjects aged 65 and over respond differently than younger subjects; however, age over 65 may be associated with a greater incidence of central nervous system depression and more respiratory depression (see WARNINGS, Preanesthetic Use, PRECAUTIONS, General and ADVERSE REACTIONS, Preanesthetic).
Age does not appear to have a clinically significant effect on lorazepam kinetics (see CLINICAL PHARMACOLOGY).
Clinical circumstances, some of which may be more common in the elderly, such as hepatic or renal impairment, should be considered. Greater sensitivity (e.g., sedation) of some older individuals cannot be ruled out. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range (see DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
Status Epilepticus - The most important adverse clinical event caused by the use of Lorazepam Injection is respiratory depression (see WARNINGS). The adverse clinical events most commonly ...
Status Epilepticus
The most important adverse clinical event caused by the use of Lorazepam Injection is respiratory depression (see WARNINGS).
The adverse clinical events most commonly observed with the use of Lorazepam Injection in clinical trials evaluating its use in status epilepticus were hypotension, somnolence, and respiratory failure.
INCIDENCE IN CONTROLLED CLINICAL TRIALS
All adverse events were recorded during the trials by the clinical investigators using terminology of their own choosing. Similar types of events were grouped into standardized categories using modified COSTART dictionary terminology. These categories are used in the table and listings below with the frequencies representing the proportion of individuals exposed to Lorazepam Injection or to comparative therapy.
The prescriber should be aware that these figures cannot be used to predict the frequency of adverse events in the course of usual medical practice where patient characteristics and other factors may differ from those prevailing during clinical studies. Similarly, the cited frequencies cannot be directly compared with figures obtained from other clinical investigators involving different treatment, uses, or investigators. An inspection of these frequencies, however, does provide the prescribing physician with one basis to estimate the relative contribution of drug and nondrug factors to the adverse event incidences in the population studied.
COMMONLY OBSERVED ADVERSE EVENTS IN A CONTROLLED DOSE-COMPARISON CLINICAL TRIAL
Table 1 lists the treatment-emergent adverse events that occurred in the patients treated with Lorazepam Injection in a dose-comparison trial of Lorazepam Injection 1 mg, 2 mg, and 4 mg.
TABLE 1. NUMBER (%) OF STUDY EVENTS IN A DOSE COMPARISON CLINICAL TRIAL a: One hundred and thirty (130) patients received Lorazepam Injection.
b: Totals are not necessarily the sum of the individual study events because a patient may report two or more different study events in the same body system.
Body System
EventLorazepam Injection
(n=130)aAny Study Event (1 or more)b 16 (12.3%) Body as a whole Infection 1 (<1%) Cardiovascular system Hypotension 2 (1.5%) Digestive system Liver function tests abnormal 1 (<1%) Nausea 1 (<1%) Vomiting 1 (<1%) Metabolic and Nutritional Acidosis 1 (<1%) Nervous system Brain edema 1 (<1%) Coma 1 (<1%) Convulsion 1 (<1%) Somnolence 2 (1.5%) Thinking abnormal 1 (<1%) Respiratory system Hyperventilation 1 (<1%) Hypoventilation 1 (<1%) Respiratory failure 2 (1.5%) Terms not classifiable Injection site reaction 1 (<1%) Urogenital system Cystitis 1 (<1%) COMMONLY OBSERVED ADVERSE EVENTS IN ACTIVE-CONTROLLED CLINICAL TRIALS
In two studies, patients who completed the course of treatment for status epilepticus were permitted to be reenrolled and to receive treatment for a second status episode, given that there was a sufficient interval between the two episodes. Safety was determined from all treatment episodes for all intent-to-treat patients, i.e., from all “patient-episodes.” Table 2 lists the treatment-emergent adverse events that occurred in at least 1% of the patient-episodes in which Lorazepam Injection or diazepam was given. The table represents the pooling of results from the two controlled trials.
TABLE 2. NUMBER (%) OF STUDY EVENTS IN ACTIVE CONTROLLED CLINICAL TRIAL a: The number indicates the number of “patient-episodes.” Patient-episodes were used rather than “patients” because a total of 7 patients were reenrolled for the treatment of a second episode of status: 5 patients received Lorazepam Injection on two occasions that were far enough apart to establish the diagnosis of status epilepticus for each episode, and, using the same time criterion, 2 patients received diazepam on two occasions.
b: Totals are not necessarily the sum of the individual study events because a patient may report two or more different study events in the same body system.
Body System Lorazepam Injection Diazepam Event (n=85)a (n=80)a Any Study Event (1 or more)b 14 (16.5%) 11 (13.8%) Body as a whole Headache 1 ( 1.2%) 1 (1.3%) Cardiovascular system Hypotension 2 (2.4%) 0 Hemic and lymphatic system Hypochromic anemia 0 1 (1.3%) Leukocytosis 0 1 (1.3%) Thrombocythemia 0 1 (1.3%) Nervous system Coma 1 (1.2 %) 1 (1.3%) Somnolence 3 (3.5%) 3 (3.8%) Stupor 1 (1.2%) 0 Respiratory system Hypoventilation 1 (1.2%) 2 (2.5%) Apnea 1 (1.2%) 1 (1.3%) Respiratory failure 2 (2.4%) 1 (1.3%) Respiratory disorder 1 (1.2%) 0 These trials were not designed or intended to demonstrate the comparative safety of the two treatments.
The overall adverse experience profile for Lorazepam Injection was similar between women and men. There are insufficient data to support a statement regarding the distribution of adverse events by race. Generally, age greater than 65 years may be associated with a greater incidence of central-nervous-system depression and more respiratory depression.
OTHER EVENTS OBSERVED DURING THE PRE-MARKETING EVALUATION OF LORAZEPAM INJECTION FOR THE TREATMENT OF STATUS EPILEPTICUS
Lorazepam Injection, active comparators, and Lorazepam Injection in combination with a comparator were administered to 488 individuals during controlled and open-label clinical trials. Because of reenrollments, these 488 patients participated in a total of 521 patient-episodes. Lorazepam Injection alone was given in 69% of these patient-episodes (n=360). The safety information below is based on data available from 326 of these patient-episodes in which Lorazepam Injection was given alone.
All adverse events that were seen once are listed, except those already included in previous listings (Table 1 and Table 2).
Study events were classified by body system in descending frequency by using the following definitions: frequent adverse events were those that occurred in at least 1/100 individuals; infrequent study events were those that occurred in 1/100 to 1/1000 individuals.
Frequent and Infrequent Study Events
BODY AS A WHOLE - Infrequent: asthenia, chills, headache, infection. DIGESTIVE SYSTEM - Infrequent: abnormal liver function test, increased salivation, nausea, vomiting. METABOLIC AND NUTRITIONAL - Infrequent: acidosis, alkaline phosphatase increased. NERVOUS SYSTEM - Infrequent: agitation, ataxia, brain edema, coma, confusion, convulsion, hallucinations, myoclonus, stupor, thinking abnormal, tremor. RESPIRATORY SYSTEM - Frequent: apnea; Infrequent: hyperventilation, hypoventilation, respiratory disorder. TERMS NOT CLASSIFIABLE - Infrequent: injection site reaction. UROGENITAL SYSTEM - Infrequent: cystitis. Preanesthetic
CENTRAL NERVOUS SYSTEM
The most frequent adverse drug event reported with injectable lorazepam is central-nervous-system depression. The incidence varied from one study to another, depending on the dosage, route of administration, use of other central-nervous-system depressants, and the investigator's opinion concerning the degree and duration of desired sedation. Excessive sleepiness and drowsiness were the most common consequences of CNS depression. This interfered with patient cooperation in approximately 6% (25/446) of patients undergoing regional anesthesia, causing difficulty in assessing levels of anesthesia. Patients over 50 years of age had a higher incidence of excessive sleepiness or drowsiness when compared with those under 50 (21/106 versus 24/245) when lorazepam was given intravenously (see DOSAGE AND ADMINISTRATION). On rare occasion (3/1580) the patient was unable to give personal identification in the operating room on arrival, and one patient fell when attempting premature ambulation in the postoperative period.
Symptoms such as restlessness, confusion, depression, crying, sobbing, and delirium occurred in about 1.3% (20/1580). One patient injured himself by picking at his incision during the immediate postoperative period.
Hallucinations were present in about 1% (14/1580) of patients and were visual and self-limiting.
An occasional patient complained of dizziness, diplopia and/or blurred vision. Depressed hearing was infrequently reported during the peak-effect period.
An occasional patient had a prolonged recovery room stay, either because of excessive sleepiness or because of some form of inappropriate behavior. The latter was seen most commonly when scopolamine was given concomitantly as a premedicant. Limited information derived from patients who were discharged the day after receiving injectable lorazepam showed one patient complained of some unsteadiness of gait and a reduced ability to perform complex mental functions. Enhanced sensitivity to alcoholic beverages has been reported more than 24 hours after receiving injectable lorazepam, similar to experience with other benzodiazepines.
LOCAL EFFECTS
Intramuscular injection of lorazepam has resulted in pain at the injection site, a sensation of burning, or observed redness in the same area in a very variable incidence from one study to another. The overall incidence of pain and burning in patients was about 17% (146/859) in the immediate postinjection period and about 1.4% (12/859) at the 24-hour observation time. Reactions at the injection site (redness) occurred in approximately 2% (17/859) in the immediate postinjection period and were present 24 hours later in about 0.8% (7/859).
Intravenous administration of lorazepam resulted in painful responses in 13/771 patients or approximately 1.6% in the immediate postinjection period, and 24 hours later 4/771 patients or about 0.5% still complained of pain. Redness did not occur immediately following intravenous injection but was noted in 19/771 patients at the 24-hour observation period. This incidence is similar to that observed with an intravenous infusion before lorazepam is given. Intra-arterial injection may produce arteriospasm resulting in gangrene which may require amputation (see CONTRAINDICATIONS).
CARDIOVASCULAR SYSTEM
Hypertension (0.1%) and hypotension (0.1%) have occasionally been observed after patients have received injectable lorazepam.
RESPIRATORY SYSTEM
Five patients (5/446) who underwent regional anesthesia were observed to have airway obstruction. This was believed due to excessive sleepiness at the time of the procedure and resulted in temporary hypoventilation. In this instance, appropriate airway management may become necessary (see CLINICAL PHARMACOLOGY, WARNINGS, and PRECAUTIONS).
Paradoxical Reactions
As with all benzodiazepines, paradoxical reactions such as stimulation, mania, irritability, restlessness, agitation, aggression, psychosis, hostility, rage, or hallucinations may occur in rare instances and in an unpredictable fashion. In these instances, further use of the drug in these patients should be considered with caution (see PRECAUTIONS, General).
ClosePostmarketing Reports
Voluntary reports of other adverse events temporally associated with the use of Lorazepam Injection that have been received since market introduction and that may have no causal relationship with the use of Lorazepam Injection include the following: acute brain syndrome, aggravation of pheochromocytoma, amnesia, apnea/respiratory arrest, arrhythmia, bradycardia, brain edema, coagulation disorder, coma, convulsion, gastrointestinal hemorrhage, heart arrest/failure, heart block, liver damage, lung edema, lung hemorrhage, nervousness, neuroleptic malignant syndrome, paralysis, pericardial effusion, pneumothorax, pulmonary hypertension, tachycardia, thrombocytopenia, urinary incontinence, ventricular arrhythmia.
Fatalities also have been reported, usually in patients on concomitant medications (e.g., respiratory depressants) and/or with other medical conditions (e.g., obstructive sleep apnea).
-
DRUG ABUSE AND DEPENDENCE
Controlled Substance - Lorazepam Injection contains lorazepam, a Schedule IV controlled substance. Abuse - Lorazepam Injection is a benzodiazepine and a CNS depressant with a potential for ...
Abuse
Lorazepam Injection is a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse of benzodiazepines may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders (see WARNINGS, Abuse, Misuse, and Addiction).
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
CloseDependence
PHYSICAL DEPENDENCE AFTER USE OF LORAZEPAM INJECTION MORE FREQUENTLY THAN RECOMMENDED
Lorazepam Injection may produce physical dependence if used more frequently than recommended. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Although Lorazepam Injection is indicated only for intermittent use (see INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION), if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening.
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use (see WARNINGS, Dependence AND Withdrawal Reactions).
For patients using Lorazepam Injection more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue Lorazepam Injection (see WARNINGS, Dependence and Withdrawal Reactions).
Acute Withdrawal Signs and Symptoms
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizures, and suicidality.
Protracted Withdrawal Syndrome
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there may be difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used.
TOLERANCE
Tolerance to Lorazepam Injection may develop after use more frequently than recommended. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose).
Tolerance to the therapeutic effect of benzodiazepines may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines.
-
OVERDOSAGE
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion ...
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal (see WARNINGS, Abuse, Misuse, and Addiction). Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway management. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil use may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil Injection Prescribing Information.
Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
Close -
DOSAGE AND ADMINISTRATION
NOTE: CONTAINS BENZYL ALCOHOL (see WARNINGS and PRECAUTIONS, Pediatric Use). Lorazepam Injection must never be used without individualization of dosage particularly when used with other ...
NOTE: CONTAINS BENZYL ALCOHOL (see WARNINGS and PRECAUTIONS, Pediatric Use).
Lorazepam Injection must never be used without individualization of dosage particularly when used with other medications capable of producing central-nervous-system depression.
EQUIPMENT NECESSARY TO MAINTAIN A PATENT AIRWAY SHOULD BE IMMEDIATELY AVAILABLE PRIOR TO INTRAVENOUS ADMINISTRATION OF LORAZEPAM (see WARNINGS).
Status Epilepticus
GENERAL ADVICE
Status epilepticus is a potentially life-threatening condition associated with a high risk of permanent neurological impairment, if inadequately treated. The treatment of status, however, requires far more than the administration of an anticonvulsant agent. It involves observation and management of all parameters critical to maintaining vital function and the capacity to provide support of those functions as required. Ventilatory support must be readily available. The use of benzodiazepines, like Lorazepam Injection, is ordinarily only an initial step of a complex and sustained intervention which may require additional interventions, (e.g., concomitant intravenous administration of phenytoin). Because status epilepticus may result from a correctable acute cause such as hypoglycemia, hyponatremia, or other metabolic or toxic derangement, such an abnormality must be immediately sought and corrected. Furthermore, patients who are susceptible to further seizure episodes should receive adequate maintenance antiepileptic therapy.
Any healthcare professional who intends to treat a patient with status epilepticus should be familiar with this package insert and the pertinent medical literature concerning current concepts for the treatment of status epilepticus. A comprehensive review of the considerations critical to the informed and prudent management of status epilepticus cannot be provided in drug product labeling. The archival medical literature contains many informative references on the management of status epilepticus, among them the report of the working group on status epilepticus of the Epilepsy Foundation of America “Treatment of Convulsive Status Epilepticus” (JAMA 1993; 270:854-859). As noted in the report just cited, it may be useful to consult with a neurologist if a patient fails to respond (e.g., fails to regain consciousness).
INTRAVENOUS INJECTION
For the treatment of status epilepticus, the usual recommended dose of Lorazepam Injection is 4 mg given slowly (2 mg/min) for patients 18 years and older. If seizures cease, no additional Lorazepam Injection is required. If seizures continue or recur after a 10- to 15-minute observation period, an additional 4 mg intravenous dose may be slowly administered. Experience with further doses of Lorazepam Injection is very limited. The usual precautions in treating status epilepticus should be employed. An intravenous infusion should be started, vital signs should be monitored, an unobstructed airway should be maintained, and artificial ventilation equipment should be available.
INTRAMUSCULAR INJECTION
Intramuscular Lorazepam Injection is not preferred in the treatment of status epilepticus because therapeutic lorazepam levels may not be reached as quickly as with intravenous administration. However, when an intravenous port is not available, the intramuscular route may prove useful (see CLINICAL PHARMACOLOGY, Pharmacokinetics and Metabolism).
Preanesthetic
INTRAMUSCULAR INJECTION
For the designated indications as a premedicant, the usual recommended dose of lorazepam for intramuscular injection is 0.05 mg/kg up to a maximum of 4 mg. As with all premedicant drugs, the dose should be individualized (see CLINICAL PHARMACOLOGY, WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS). Doses of other central-nervous-system-depressant drugs ordinarily should be reduced (see PRECAUTIONS). For optimum effect, measured as lack of recall, intramuscular lorazepam should be administered at least 2 hours before the anticipated operative procedure. Narcotic analgesics should be administered at their usual preoperative time.
There are insufficient data to support efficacy or make dosage recommendations for intramuscular lorazepam in patients less than 18 years of age; therefore, such use is not recommended.
INTRAVENOUS INJECTION
For the primary purpose of sedation and relief of anxiety, the usual recommended initial dose of lorazepam for intravenous injection is 2 mg total, or 0.02 mg/lb (0.044 mg/kg), whichever is smaller. This dose will suffice for sedating most adult patients and ordinarily should not be exceeded in patients over 50 years of age. In those patients in whom a greater likelihood of lack of recall for perioperative events would be beneficial, larger doses as high as 0.05 mg/kg up to a total of 4 mg may be administered (see CLINICAL PHARMACOLOGY, WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS). Doses of other injectable central-nervous-system-depressant drugs ordinarily should be reduced (see PRECAUTIONS).
For optimum effect, measured as lack of recall, intravenous lorazepam should be administered 15 to 20 minutes before the anticipated operative procedure.
There are insufficient data to support efficacy or make dosage recommendations for intravenous lorazepam in patients less than 18 years of age; therefore, such use is not recommended.
Dose Administration in Special Populations
ELDERLY PATIENTS AND PATIENTS WITH HEPATIC DISEASE
No dosage adjustments are needed in elderly patients and in patients with hepatic disease.
PATIENTS WITH RENAL DISEASE
For acute dose administration, adjustment is not needed for patients with renal disease. However, in patients with renal disease, caution should be exercised if frequent doses are given over relatively short periods of time (see CLINICAL PHARMACOLOGY).
DOSE ADJUSTMENT DUE TO DRUG INTERACTIONS
The dose of Lorazepam Injection should be reduced by 50% when coadministered with probenecid or valproate (see PRECAUTIONS, Drug Interactions).
It may be necessary to increase the dose of Lorazepam Injection in female patients who are concomitantly taking oral contraceptives.
CloseAdministration
When given intramuscularly, Lorazepam Injection, undiluted, should be injected deep in the muscle mass.
Injectable Lorazepam can be used with atropine sulfate, narcotic analgesics, other parenterally used analgesics, commonly used anesthetics, and muscle relaxants.
Immediately prior to intravenous use, Lorazepam Injection must be diluted with an equal volume of compatible solution. Contents should be mixed thoroughly by gently inverting the container repeatedly until a homogenous solution results. Do not shake vigorously, as this will result in air entrapment. When properly diluted, the drug may be injected directly into a vein or into the tubing of an existing intravenous infusion.
The rate of injection should not exceed 2 mg per minute.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if solution is discolored or contains a precipitate. Discard unused portion.
Lorazepam Injection is compatible for dilution purposes with the following solutions: Sterile Water for Injection, USP; Sodium Chloride Injection, USP; 5% Dextrose Injection, USP.
-
HOW SUPPLIED
Lorazepam Injection, USP is available as: Product CodeUnit of SaleStrength - (Concentration)Each - 368302 - NDC 65219-368-02 - Unit of 25 - 2 mg/mL - NDC 65219-368-01 - 1 mL Single-Dose ...
Lorazepam Injection, USP is available as:
Product Code Unit of Sale Strength
(Concentration)Each 368302 NDC 65219-368-02
Unit of 25
2 mg/mL NDC 65219-368-01
1 mL Single-Dose Vial368310 NDC 65219-368-10
Unit of 10
20 mg per 10 mL
(2 mg/mL)NDC 65219-368-08
10 mL Multiple-Dose VialFor Intramuscular or Intravenous injection.
Store in a refrigerator. PROTECT FROM LIGHT.Use carton to protect contents from light.
The container closure is not made with natural rubber latex.
Close -
ANIMAL TOXICOLOGY AND/OR PHARMACOLOGY
Published studies in animals demonstrate that the use of anesthetic agents during the period of rapid brain growth or synaptogenesis results in widespread neuronal and oligodendrocyte cell loss in ...
Published studies in animals demonstrate that the use of anesthetic agents during the period of rapid brain growth or synaptogenesis results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester through the first several months of life, but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of an anesthetic regimen that produced a light surgical plane of anesthesia did not increase neuronal cell loss, however, treatment regimens of 5 hours or longer increased neuronal cell loss. Data in rodents and in primates suggest that the neuronal and oligodendrocyte cell losses are associated with subtle but prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in neonates and young children who require procedures against the potential risks suggested by the nonclinical data (see WARNINGS, Pediatric Neurotoxicity; PRECAUTIONS, Pregnancy, Pediatric Use).
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For Product Inquiry call 1-800-551-7176.
Manufactured by:
www.fresenius-kabi.com/us
451836
Issued: September 2024
Close -
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Lorazepam 1 mL Vial Label - NDC 65219-368-01 Rx only - Lorazepam CIV - Injection, USP - 2 mg/mL - For Intramuscular use - Dilute for Intravenous use - Protect ...
PACKAGE LABEL - PRINCIPAL DISPLAY – Lorazepam 1 mL Vial Label
NDC 65219-368-01 Rx only
Lorazepam CIV
Injection, USP
2 mg/mL
For Intramuscular use
Dilute for Intravenous use
Protect from light. Refrigerate
Discard unused portion.
1 mL Single-Dose Vial
Close -
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY – Lorazepam 1 mL Tray Label - NDC 65219-368-02 Rx only - Lorazepam CIV - Injection, USP - 2 mg/mL - For Intramuscular use - For Intravenous use ...
PACKAGE LABEL - PRINCIPAL DISPLAY – Lorazepam 1 mL Tray Label
NDC 65219-368-02 Rx only
Lorazepam CIV
Injection, USP
2 mg/mL
For Intramuscular use
For Intravenous use, dilution
required; see insert.
Refrigerate
Discard unused portion.
25 x 1 mL Single-Dose Vials
Close -
INGREDIENTS AND APPEARANCEProduct Information
LORAZEPAM lorazepam injection Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65219-368 Route of Administration INTRAMUSCULAR, INTRAVENOUS DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORAZEPAM (UNII: O26FZP769L) (LORAZEPAM - UNII:O26FZP769L) LORAZEPAM 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65219-368-02 25 in 1 TRAY 12/13/2024 1 NDC:65219-368-01 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA217598 12/13/2024 Labeler - Fresenius Kabi USA, LLC (013547657)
CloseEstablishment Name Address ID/FEI Business Operations Fresenius Kabi USA, LLC 840771732 ANALYSIS(65219-368) , MANUFACTURE(65219-368)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
LORAZEPAM injection
Number of versions: 1
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Dec 13, 2024 | 1 (current) | download |
Get Label RSS Feed for this Drug
LORAZEPAM injection
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=b2487467-a63f-48b5-b0de-3a6ce6672d50
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
LORAZEPAM injection
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 65219-368-01 |
| 2 | 65219-368-02 |