Label: TRELSTAR- triptorelin pamoate kit
- NDC Code(s): 74676-5902-0, 74676-5902-1, 74676-5904-0, 74676-5904-1, view more
- Packager: Verity Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TRELSTAR safely and effectively. See full prescribing information for TRELSTAR. TRELSTAR® (triptorelin pamoate for injectable ...
-
Table of ContentsTable of Contents
-
1
INDICATIONS AND USAGE
TRELSTAR is indicated for the treatment of advanced prostate cancer [see Clinical Studies (14)].
-
2
DOSAGE AND ADMINISTRATION
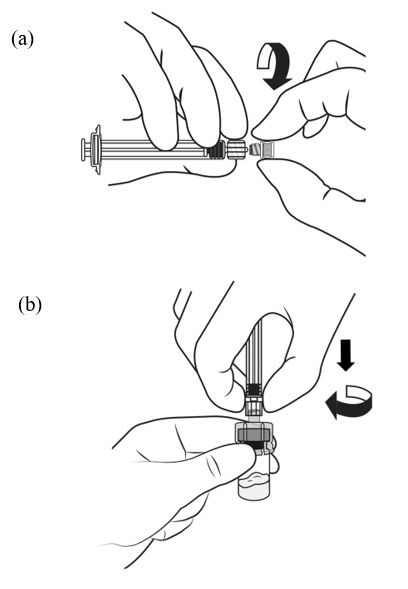
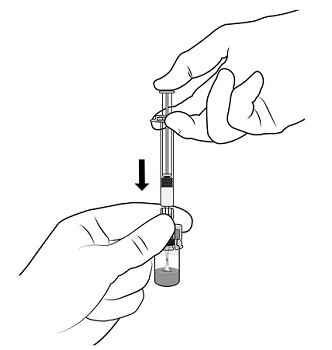
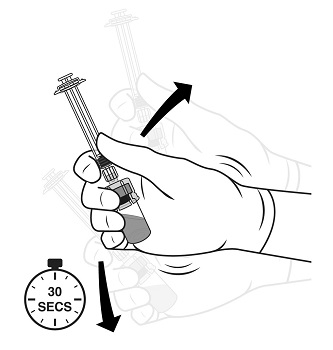
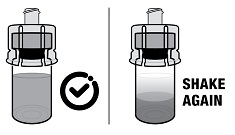
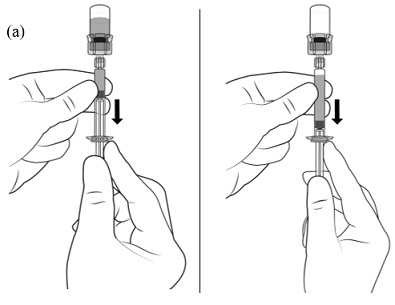
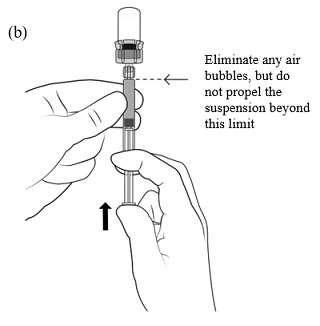
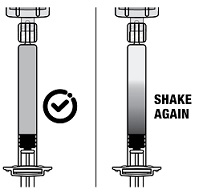
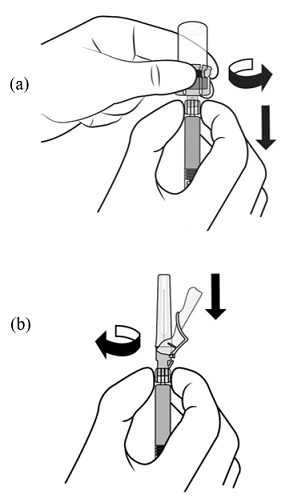
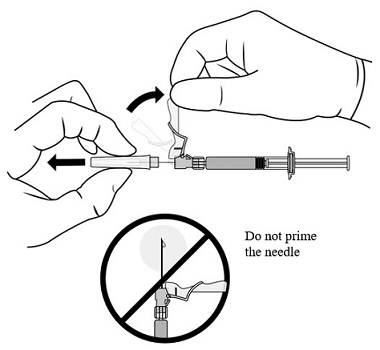
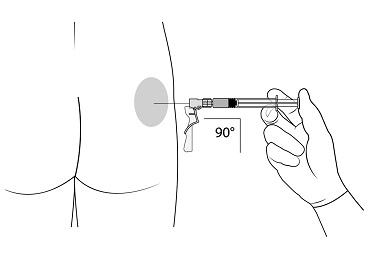
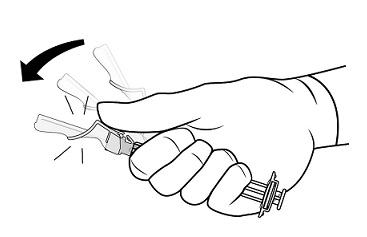
2.1 Dosing Information - TRELSTAR must be administered under the supervision of a physician. TRELSTAR is administered by a single intramuscular injection in either buttock. Dosing ...
-
3
DOSAGE FORMS AND STRENGTHS
For injectable suspension: 3.75 mg, 11.25 mg, 22.5 mg of slightly yellow lyophilized microgranules in a single-dose vial for reconstitution with an injection kit containing one syringe filled with ...
-
4
CONTRAINDICATIONS
4.1 Hypersensitivity - TRELSTAR is contraindicated in individuals with a known hypersensitivity to triptorelin or any other component of the product, or other GnRH agonists or GnRH [see ...
-
5
WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions - Anaphylactic shock, hypersensitivity, and angioedema related to TRELSTAR administration have been reported. In the event of a hypersensitivity reaction ...
-
6
ADVERSE REACTIONS
The following is discussed in more detail in other sections of the labeling: Hypersensitivity Reactions [see Warnings and Precautions (5.1)] Tumor Flare [see Warnings and Precautions ...
-
7
DRUG INTERACTIONS
No drug-drug interaction studies involving TRELSTAR have been conducted. Human pharmacokinetic data with triptorelin suggest that C-terminal fragments produced by tissue degradation are either ...
-
8
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Based on findings in animal studies and mechanism of action, TRELSTAR can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology ...
-
11
DESCRIPTION
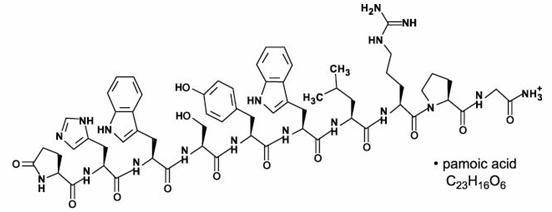
TRELSTAR is a white to slightly yellow lyophilized cake. When reconstituted, TRELSTAR has a milky appearance. It contains a pamoate salt of triptorelin, a synthetic decapeptide agonist analog of ...
-
12
CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Triptorelin is a synthetic decapeptide agonist analog of gonadotropin releasing hormone (GnRH). Comparative in vitro studies showed that triptorelin was ...
-
13
NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In rats, triptorelin doses of 120, 600, and 3000 mcg/kg given every 28 days (approximately 0.3, 2, and 8 times the human ...
-
14
CLINICAL STUDIES
TRELSTAR 3.75 mg - TRELSTAR 3.75 mg was studied in a randomized, active control trial of 277 men with advanced prostate cancer. The clinical trial population consisted of 59.9% Caucasian, 39.3 ...
-
16
HOW SUPPLIED/STORAGE AND HANDLING
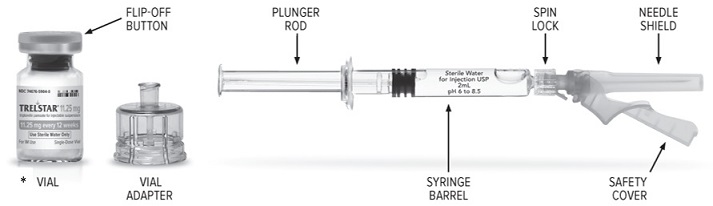
TRELSTAR (triptorelin pamoate for injectable suspension) is supplied as a single dose vial with a Flip-Off cap containing sterile lyophilized triptorelin pamoate microgranules incorporated in a ...
-
17
PATIENT COUNSELING INFORMATION
Hypersensitivity - Inform patients that if they have experienced hypersensitivity with other GnRH agonist drugs like TRELSTAR, TRELSTAR is contraindicated [see Contraindications (4)]. Tumor ...
-
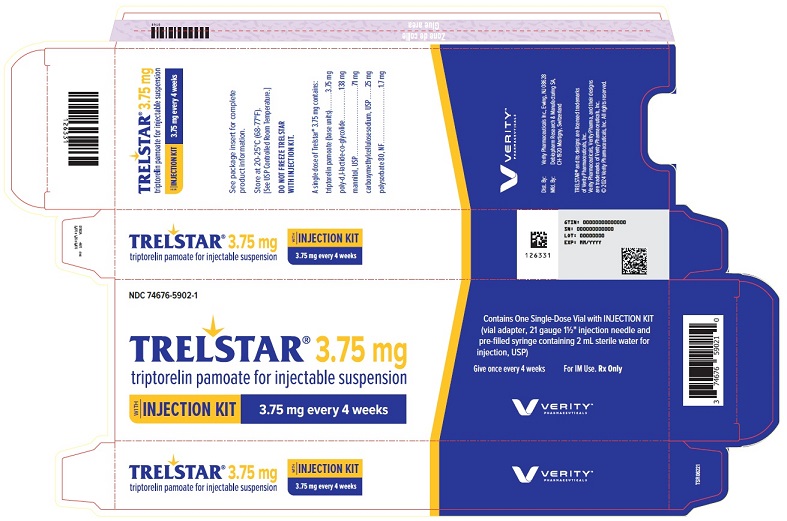
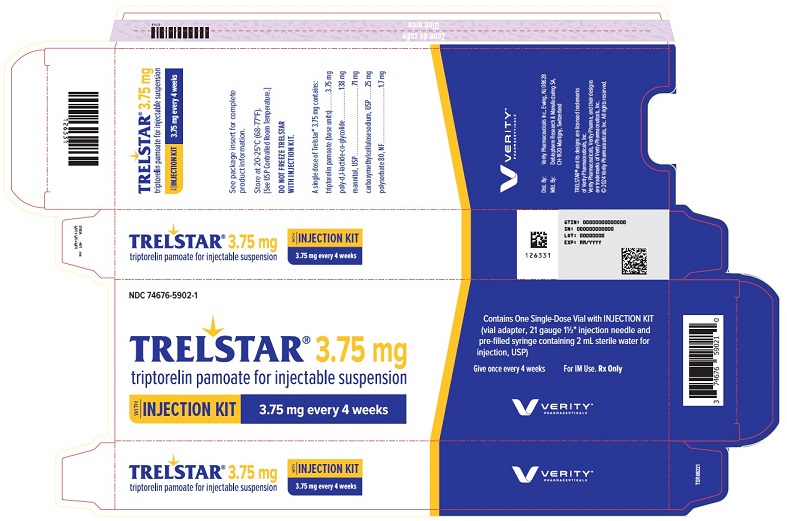
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - Trelstar 3.75 mg - 3.75 mg every 4 weeks
-
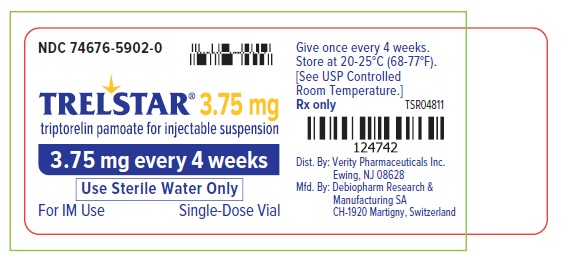
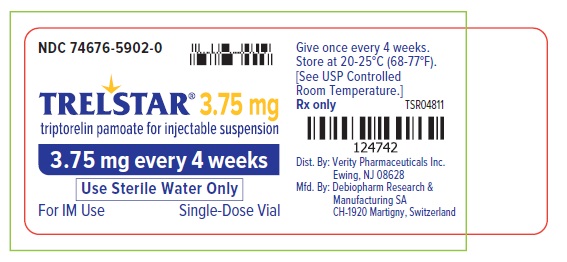
PRINCIPAL DISPLAY PANELTrelstar 3.75 mg Vial
-
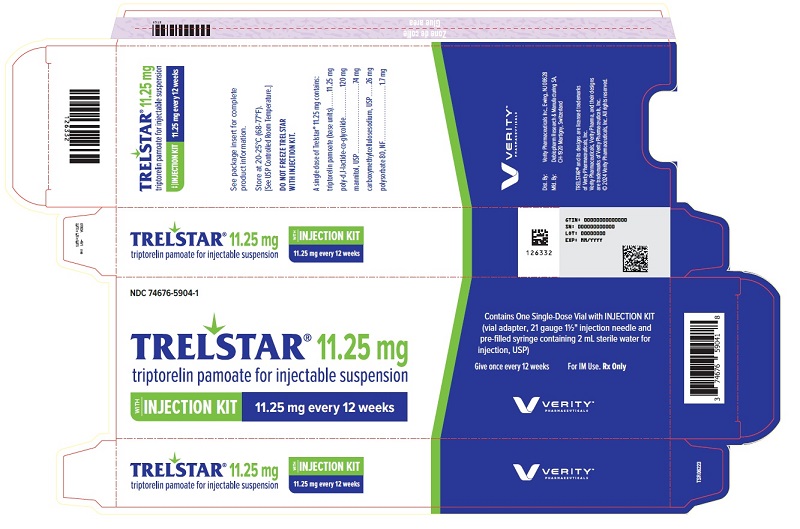
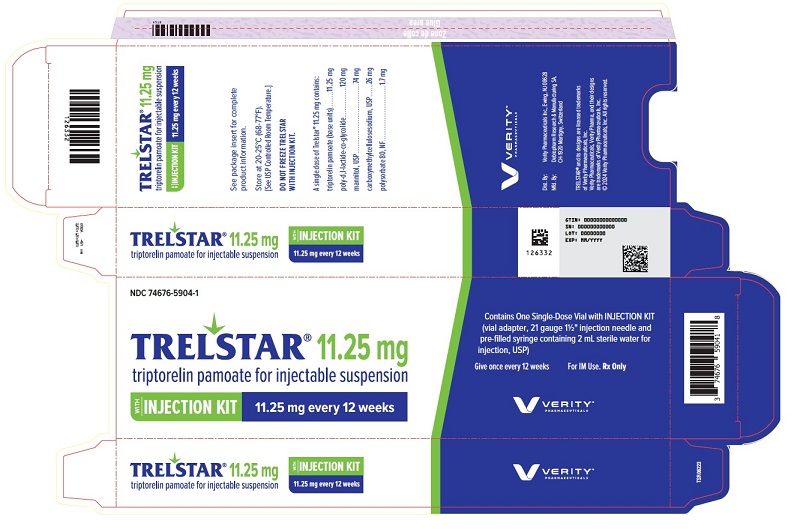
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - Trelstar 11.25 mg - 11.25 mg every 12 weeks
-
PRINCIPAL DISPLAY PANELTrelstar 11.25 mg Vial
-
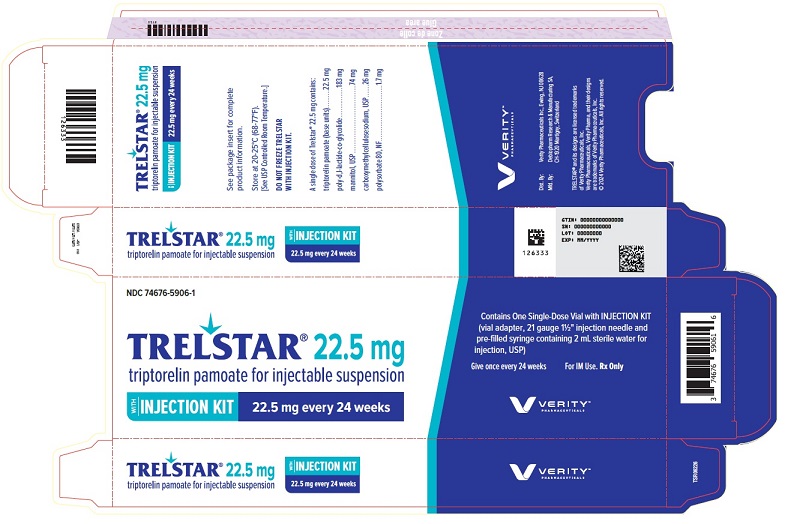
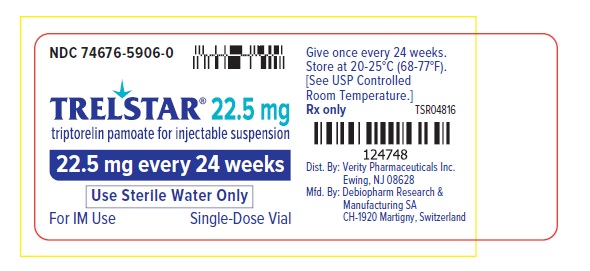
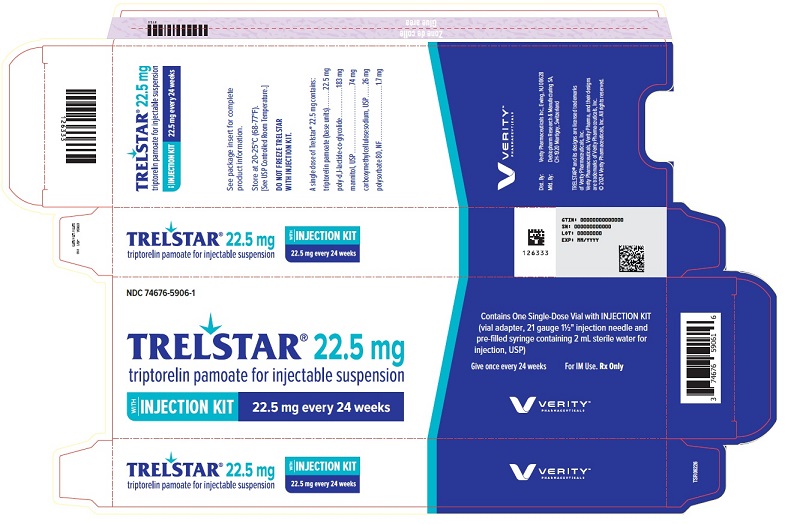
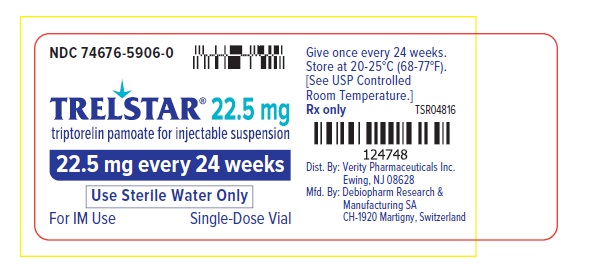
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - Trelstar 22.5 mg - 22.5 mg every 24 weeks
-
PRINCIPAL DISPLAY PANELTrelstar 22.5 mg Vial
-
INGREDIENTS AND APPEARANCEProduct Information