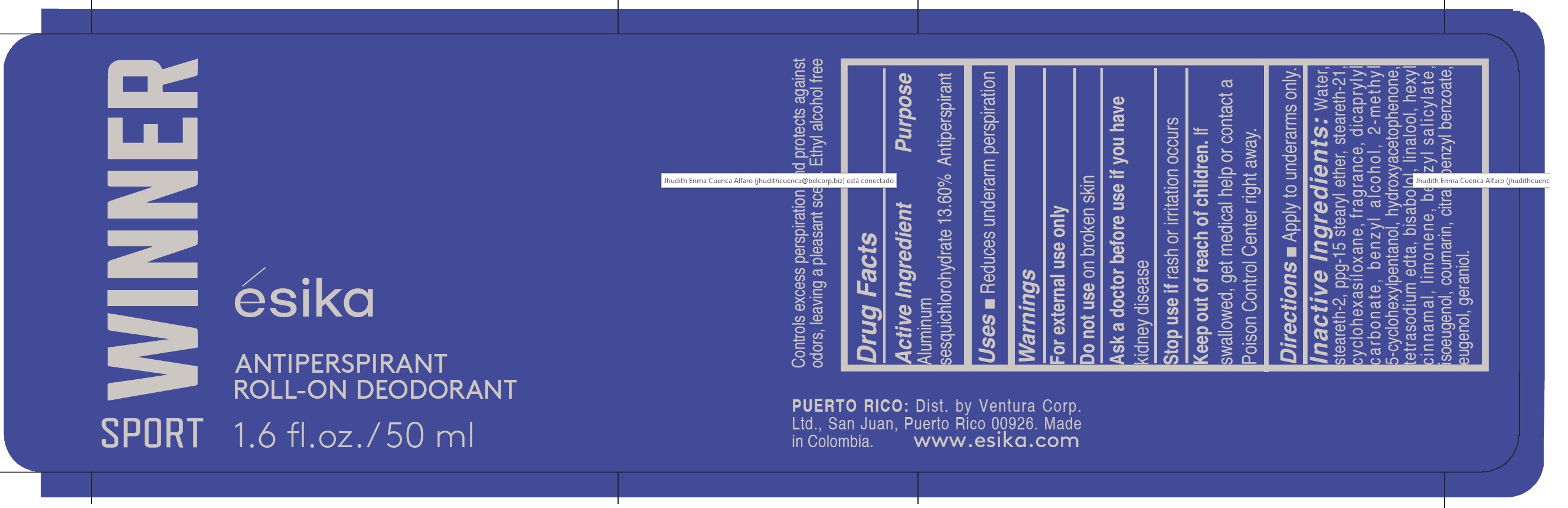
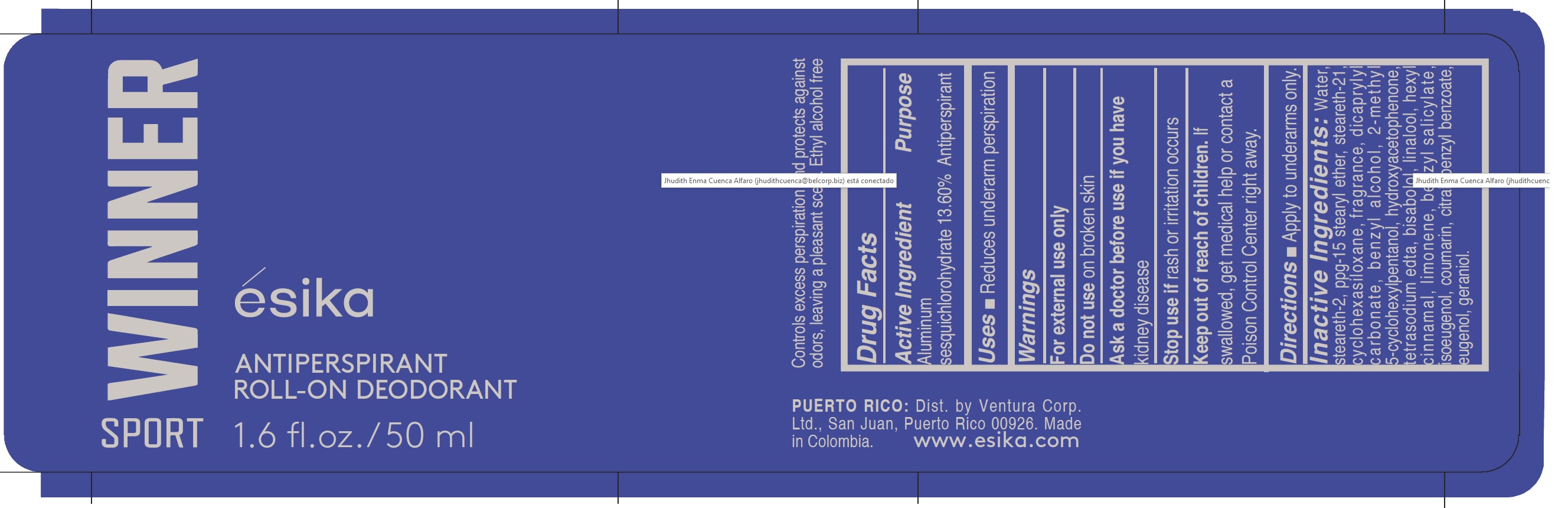
Label: ESIKA WINNER SPORT ANTIPERSPIRANT ROLL-ON DEODORANT- aluminum sesquichlorohydrate emulsion
- NDC Code(s): 14141-200-01
- Packager: Bel Star S.A. (Colombia)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 16, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients:
water, steareth-2,ppg-15 stearyl ether, steareth-21, cyclohexasiloxane, fragrance, dicaprylyl carbonate, benzyl alcohol, 2-methyl 5-cyclohexylpentanol, hydroxyacetophenone, tetrasodium edta, bisabolol, linalool, hexyl cinnamal, limonene, benzyl salicylate, isoeugenol, coumarin, citral, benzyl benzoate, eugenol, geraniol.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ESIKA WINNER SPORT ANTIPERSPIRANT ROLL-ON DEODORANT
aluminum sesquichlorohydrate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14141-200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM SESQUICHLOROHYDRATE (UNII: UCN889409V) (ALUMINUM SESQUICHLOROHYDRATE - UNII:UCN889409V) ALUMINUM SESQUICHLOROHYDRATE 13.6 g in 100 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) STEARETH-2 (UNII: V56DFE46J5) BENZYL BENZOATE (UNII: N863NB338G) EUGENOL (UNII: 3T8H1794QW) CITRAL (UNII: T7EU0O9VPP) LIMONENE, (+)- (UNII: GFD7C86Q1W) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) GERANIOL (UNII: L837108USY) BENZYL SALICYLATE (UNII: WAO5MNK9TU) ISOEUGENOL (UNII: 5M0MWY797U) COUMARIN (UNII: A4VZ22K1WT) 2-METHYL 5-CYCLOHEXYLPENTANOL (UNII: 460837ILID) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PPG-15 STEARYL ETHER (UNII: 1II18XLS1L) STEARETH-21 (UNII: 53J3F32P58) LINALOOL, (+)- (UNII: F4VNO44C09) WATER (UNII: 059QF0KO0R) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) EDETATE SODIUM (UNII: MP1J8420LU) LEVOMENOL (UNII: 24WE03BX2T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14141-200-01 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/07/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M019 10/07/2020 Labeler - Bel Star S.A. (Colombia) (880160197) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 manufacture(14141-200)