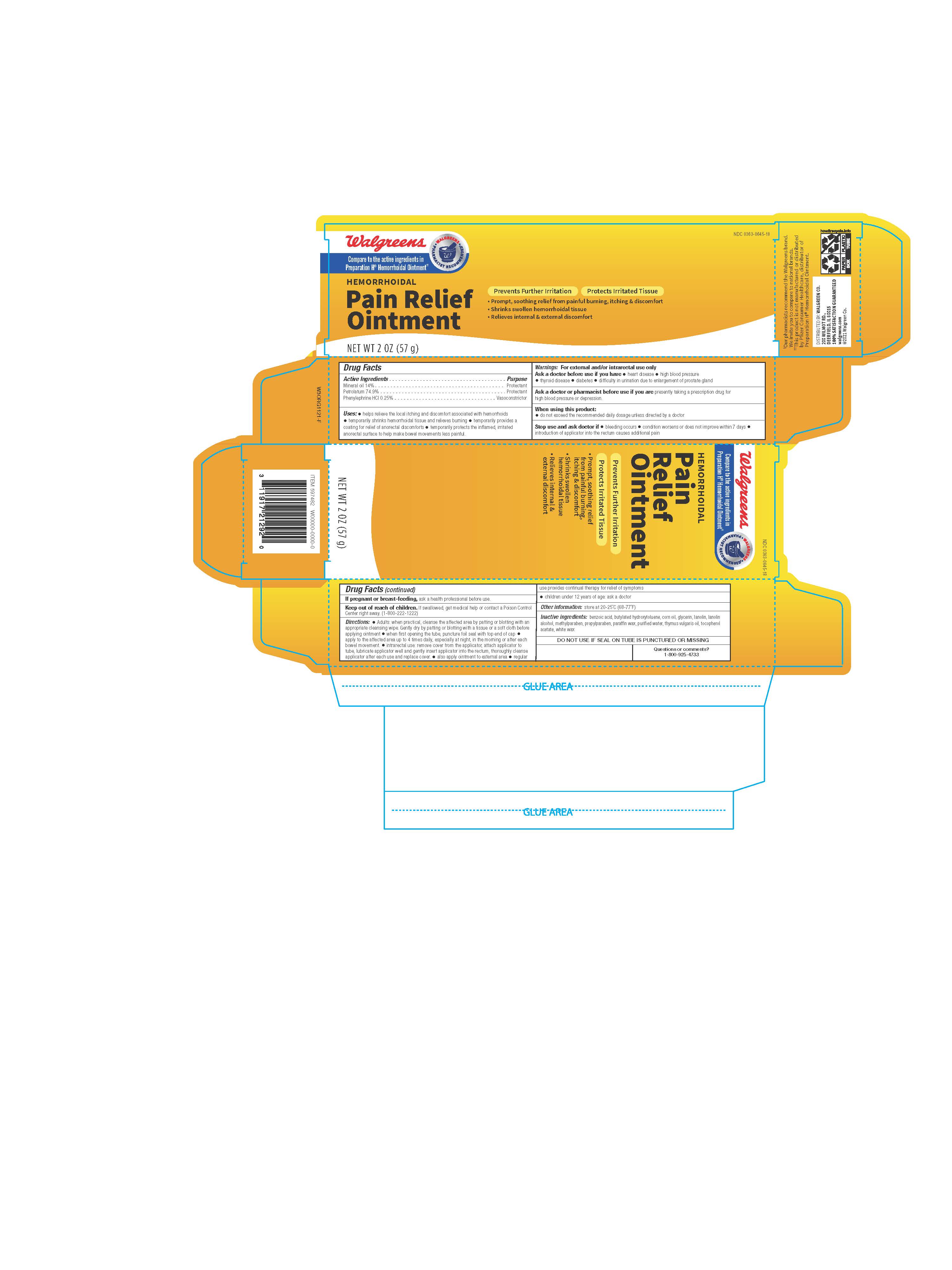
Label: WALGREENS- hemorrhoidal pain relief ointment
- NDC Code(s): 0363-6503-01, 0363-6503-02
- Packager: Walgreens
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSES
-
USES
- helps relieve the local itching and discomfort associated with hemorrhoids
- temporarily shrinks hemorrhoidal tissue and relieves burning
- temporarily provides a coating for relief of anorectal discomforts
- temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful
-
WARNINGS
For external and/or intrarectal use only
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- difficulty in urination due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug for high blood pressure or depression.
-
DIRECTIONS
- adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or a soft cloth before applying ointment.
- when first opening the tube, puncture foil seal with top end of cap
- apply to the affected area up to 4 times daily, especially at night, in the morning or after each bowel movement
- intrarectal use:
- remove cover from applicator, attach applicator to tube, lubricate applicator well and gently insert applicator into the rectum
- thoroughly cleanse applicator after each use and replace cover
- also apply ointment to external area
- regular use provides continual therapy for relief of symptoms
- children under 12 years of age: ask a doctor
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS OR COMMENTS?
- 28 g Carton Label
- 28 g Tube Label
- 57 g Carton Label
- 57 g Tube Label
-
INGREDIENTS AND APPEARANCE
WALGREENS
hemorrhoidal pain relief ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-6503 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 2.5 mg in 1 g MINERAL OIL (UNII: T5L8T28FGP) (MINERAL OIL - UNII:T5L8T28FGP) MINERAL OIL 140 mg in 1 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 749 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZOIC ACID (UNII: 8SKN0B0MIM) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) CORN OIL (UNII: 8470G57WFM) GLYCERIN (UNII: PDC6A3C0OX) LANOLIN (UNII: 7EV65EAW6H) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) METHYLPARABEN (UNII: A2I8C7HI9T) PARAFFIN (UNII: I9O0E3H2ZE) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) THYME (UNII: CW657OBU4N) WHITE WAX (UNII: 7G1J5DA97F) TOCOPHEROL (UNII: R0ZB2556P8) Product Characteristics Color yellow (smooth yellow ointment) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-6503-01 1 in 1 CARTON 03/01/2004 1 28 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0363-6503-02 1 in 1 CARTON 03/01/2004 2 57 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 03/01/2004 Labeler - Walgreens (008965063) Registrant - Unipack LLC (116015769) Establishment Name Address ID/FEI Business Operations Unipack LLC 009248480 manufacture(0363-6503)