Label: PROACTIV CLEAR SKIN SPF 30- avobenzone, octisalate, and octocrylene lotion
- NDC Code(s): 11410-073-00
- Packager: Alchemee, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
-
Uses
- Helps prevent sunburn.
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
-
Sun protection measures. Spending time in the sun increases your risk of cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially 10am - 2pm
- Wear long-sleeved shirts, pants, hats and sun glasses.
- Children under 6 months; Ask a doctor.
- Other Information
-
Inactive Ingredients
Water, Isopropyl Lauroyl Sarcosinate, Glycerin, Dimethicone, Diisopropyl Sebacate, Sucrose Tristearate, Polymethyl Methacrylate, Silica, Aluminum Starch Octenylsuccinate, Pentylene Glycol, Polysorbate 61, Sodium Stearoyl Glutamate, Phenoxyethanol, Caprylyl Glycol, Tocopheryl Acetate, Dimethiconol, Glycyrrhetinic Acid, Xanthan Gum, Potassium Sorbate, Disodium EDTA, Panthenol, Carbomer, Allantoin, Triethanolamine, Zinc Gluconate, Hydroxypalmitoyl Sphinganine
- Questions?
- SPL UNCLASSIFIED SECTION
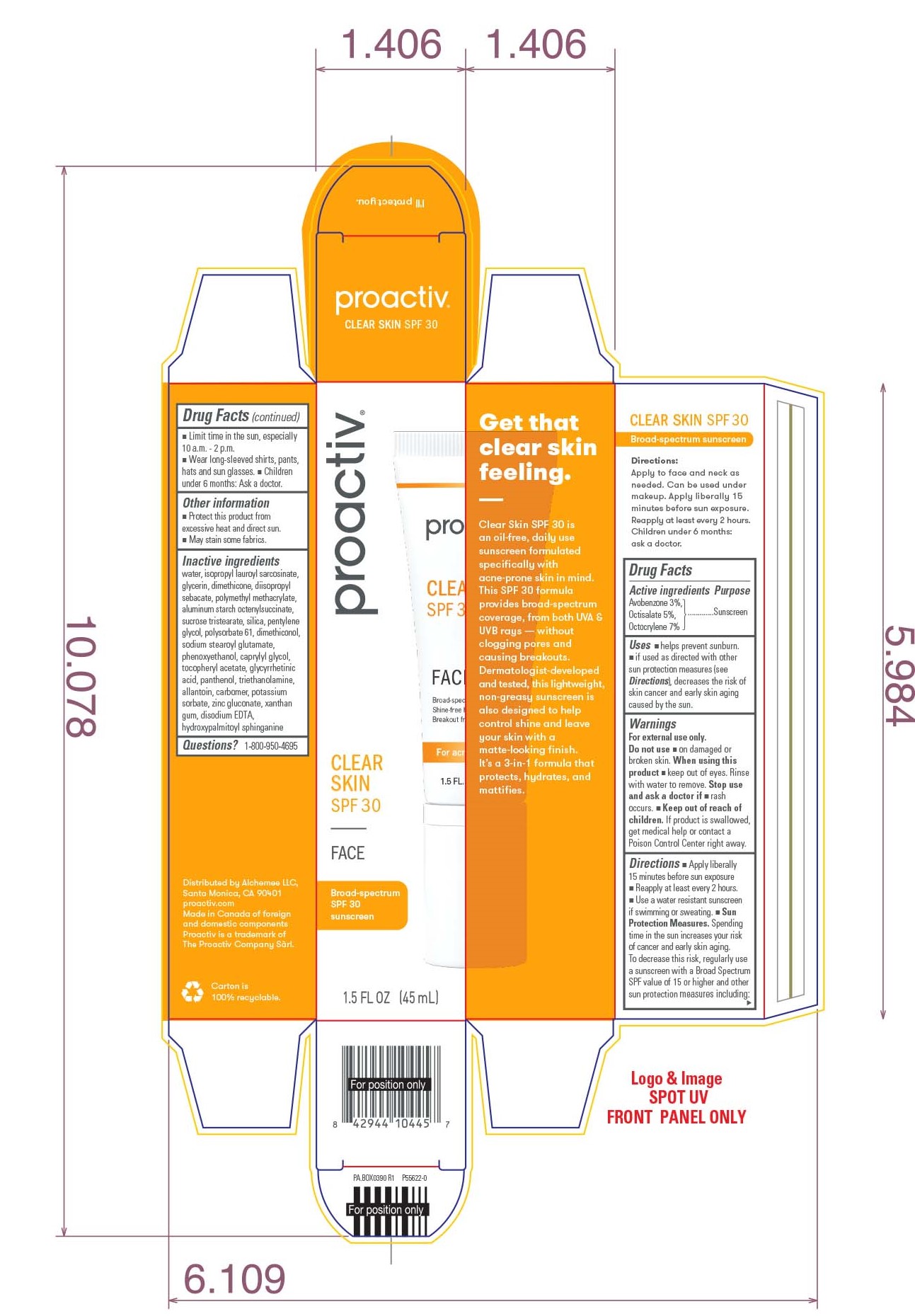
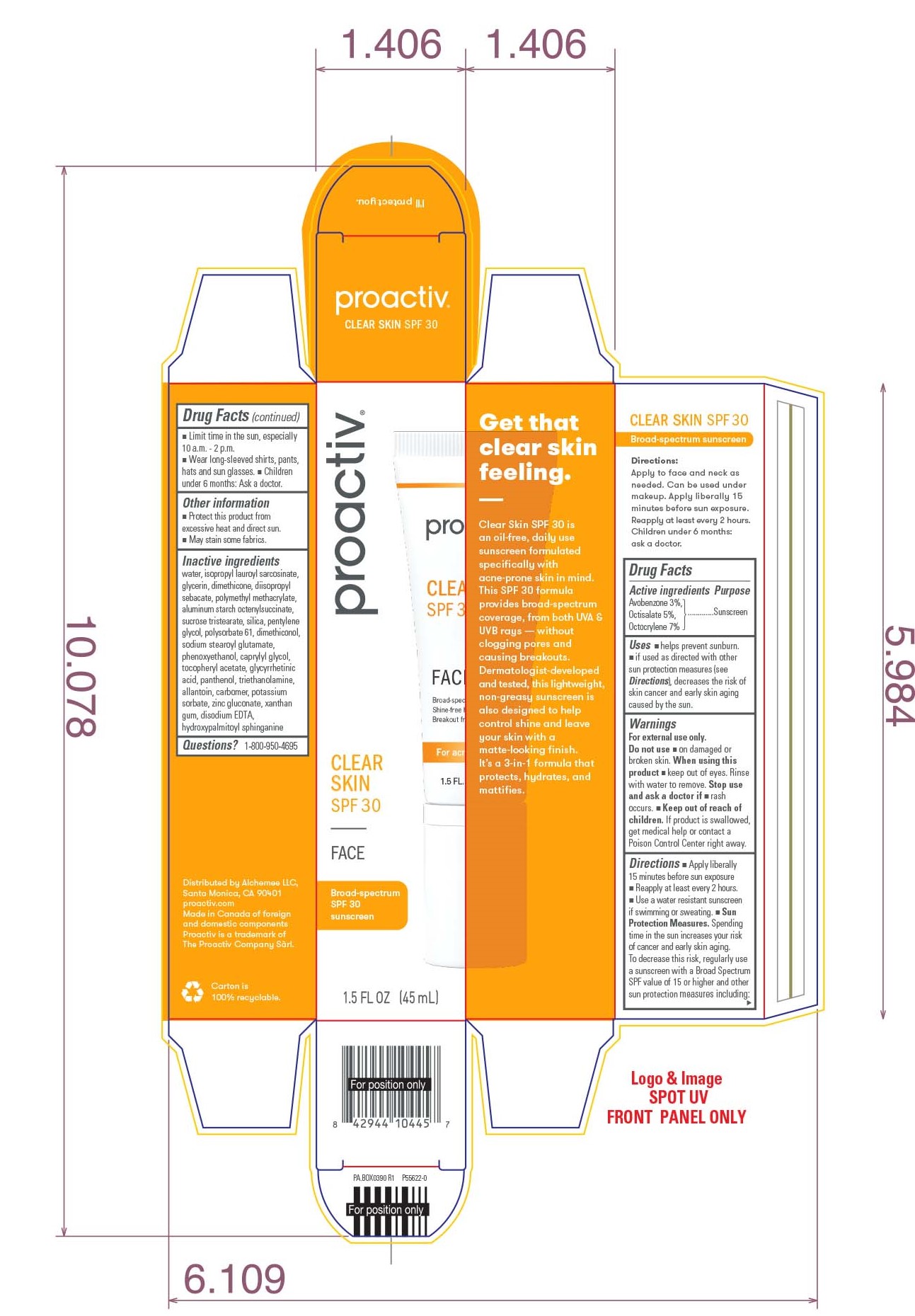
- PRINCIPAL DISPLAY PANEL - 45 ML Tube Box
-
INGREDIENTS AND APPEARANCE
PROACTIV CLEAR SKIN SPF 30
avobenzone, octisalate, and octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11410-073 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 mg in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 mg in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOPROPYL LAUROYL SARCOSINATE (UNII: LYR06W430J) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) SUCROSE TRISTEARATE (UNII: 71I93STU5M) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PENTYLENE GLYCOL (UNII: 50C1307PZG) POLYSORBATE 61 (UNII: X9E1MY2JQG) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ENOXOLONE (UNII: P540XA09DR) PANTHENOL (UNII: WV9CM0O67Z) TROLAMINE (UNII: 9O3K93S3TK) ALLANTOIN (UNII: 344S277G0Z) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ZINC GLUCONATE (UNII: U6WSN5SQ1Z) XANTHAN GUM (UNII: TTV12P4NEE) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) HYDROXYPALMITOYL SPHINGANINE (UNII: NR33W2353T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11410-073-00 1 in 1 BOX 12/13/2021 1 45 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/13/2021 Labeler - Alchemee, LLC (080216357) Establishment Name Address ID/FEI Business Operations G Production Inc. 251676961 manufacture(11410-073)