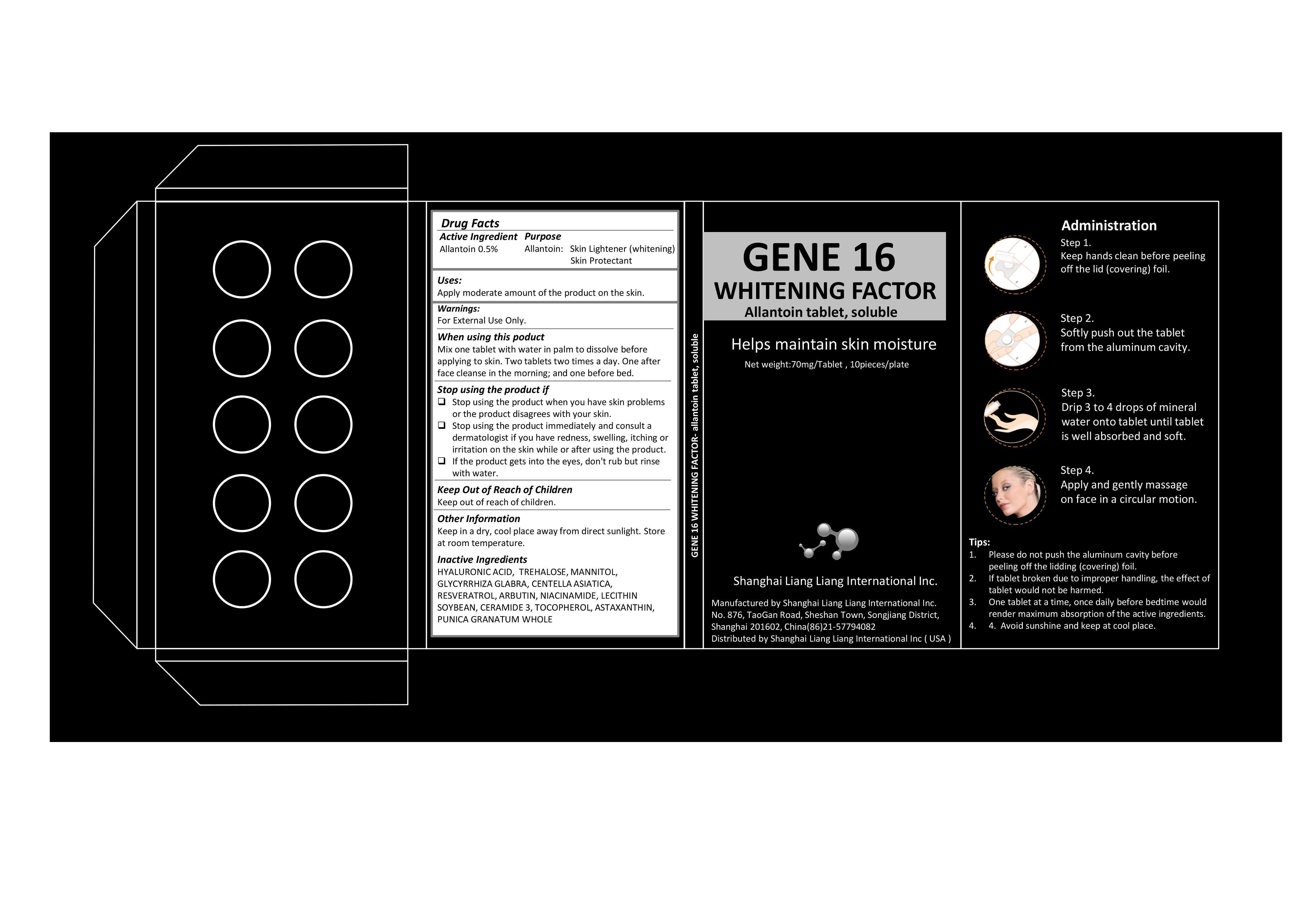
Label: GENE 16 WHITENING FACTOR- allantoin tablet, soluble
- NDC Code(s): 69764-001-10
- Packager: Shanghai Liang Liang International Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Warning
- Use
-
Stop Using the Product if
Stop using the product when you have skin problems or the product disagrees with your skin.
・Stop using the product immediately and consult a dermatologist if you have redness, swelling, itching or irritation on the skin while or after using the product.
・If the product gets into the eyes, don't rub but rinse with water.
- Keep Out of Reach of Children
- When Using the Product
- Other Information
- Contact the Manufacturer or Distributor
- Inactive Ingredients
- Drug Facts
-
INGREDIENTS AND APPEARANCE
GENE 16 WHITENING FACTOR
allantoin tablet, solubleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69764-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 0.5 mg in 100 mg Inactive Ingredients Ingredient Name Strength HYALURONIC ACID (UNII: S270N0TRQY) TREHALOSE (UNII: B8WCK70T7I) MANNITOL (UNII: 3OWL53L36A) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) CENTELLA ASIATICA (UNII: 7M867G6T1U) RESVERATROL (UNII: Q369O8926L) ARBUTIN (UNII: C5INA23HXF) NIACINAMIDE (UNII: 25X51I8RD4) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) CERAMIDE 3 (UNII: 4370DF050B) TOCOPHEROL (UNII: R0ZB2556P8) ASTAXANTHIN (UNII: 8XPW32PR7I) PUNICA GRANATUM WHOLE (UNII: O2ZTS50U5E) Product Characteristics Color white Score no score Shape OVAL Size 8mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69764-001-10 1 in 1 PACKAGE 09/25/2014 1 7 mg in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 09/25/2014 Labeler - Shanghai Liang Liang International Inc (547834726) Registrant - Shanghai Liang Liang International Inc (547834726) Establishment Name Address ID/FEI Business Operations Shanghai Liang Liang International Inc 547834726 manufacture(69764-001)